Practice Drawing Ionic Bonds
Practice Drawing Ionic Bonds - Identify the type of bond described for each of the following as ionic, polar. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions. Exercises why is an ionic compound unlikely to consist of two positively charged ions? An ionic bond occurs when a metal atom loses valence electron (s) to become a positive (+). Show the electron dot method, the crisscross method and the name of each compound. Group 2 metals can bond with group 17 nonmetals to form ionic compounds. Web if the negative ion is a single element the end is? In this game, the concept of valence is clearly demonstrated using interlocking shapes to represent cations and anions. Hcl, hf, h 2 o, nh 3, hi 13. Use the periodie table to fill in the chart below. Examples get harder as the sheet progresses. Sicl 4, pcl 3 problem \(\pageindex{3}\) for each of the following compounds, state whether it is ionic or covalent. Exercises why is an ionic compound unlikely to consist of two positively charged ions? Then answer the questions that follow. Web ionic and covalent bonding practice. Two electrons are shared in a single. Then answer the questions that follow. Web this quiz aligns with the following ngss standard (s): Sicl 4, pcl 3 problem \(\pageindex{3}\) for each of the following compounds, state whether it is ionic or covalent. Use the periodie table to fill in the chart below. Hcl, hf, h 2 o, nh 3, hi 13. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions. Each bond includes a sharing of electrons between atoms. Web if the negative. Group 2 metals can bond with group 17 nonmetals to form ionic compounds. An ionic bond occurs when a metal atom loses valence electron (s) to become a positive (+). Two electrons are shared in a single. Organize the following in order from least to most polar bonds: Ethanethiol, c a 2 h a 6 s , is a clear. Web if the negative ion is a single element the end is? An ionic bond forms between a metal and a nonmetal. Organize the following in order from least to most polar bonds: Show the electron dot method, the crisscross method and the name of each compound. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine,. An ionic bond occurs when a metal atom loses valence electron (s) to become a positive (+). A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web how are single, double, and triple bonds similar? A dot and cross diagram is one way. The compound is often added to otherwise. Hcl, hf, h 2 o, nh 3, hi 13. Exercises why is an ionic compound unlikely to consist of two positively charged ions? Web ionic and covalent bonding practice. Sicl 4, pcl 3 problem \(\pageindex{3}\) for each of the following compounds, state whether it is ionic or covalent. Play the ionic reaction simulator to learn more about the mechanics of. Characteristic properties of ionic compounds. Web ionic and covalent bonding practice. An ionic bond occurs when a metal atom loses valence electron (s) to become a positive (+). Exercises why is an ionic compound unlikely to consist of two positively charged ions? In this game, the concept of valence is clearly demonstrated using interlocking shapes to represent cations and anions. Use the periodie table to fill in the chart below. Web how are single, double, and triple bonds similar? In this game, the concept of valence is clearly demonstrated using interlocking shapes to represent cations and anions. Representing ionic solids using particulate models. Then answer the questions that follow. Use the periodie table to fill in the chart below. Hcl, hf, h 2 o, nh 3, hi 13. Play the ionic reaction simulator to learn more about the mechanics of ionic bonding at the molecular level. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross. Use the periodie table to fill in the chart below. Web ionic bonds are formed by the attraction between oppositely charged ions. Exercises why is an ionic compound unlikely to consist of two positively charged ions? Hcl, hf, h 2 o, nh 3, hi 13. Then answer the questions that follow. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Organize the following in order from least to most polar bonds: Group #of valence electrons element. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The compound is often added to otherwise. Draw the following to form ionic compounds. Identify the type of bond described for each of the following as ionic, polar. An ionic bond occurs when a metal atom loses valence electron (s) to become a positive (+). Web ionic and covalent bonding practice. Group 2 metals can bond with group 17 nonmetals to form ionic compounds. Play the ionic reaction simulator to learn more about the mechanics of ionic bonding at the molecular level.
Ionic Bonds Practice
Ionic Bonding Practice Worksheet Answers Coearth
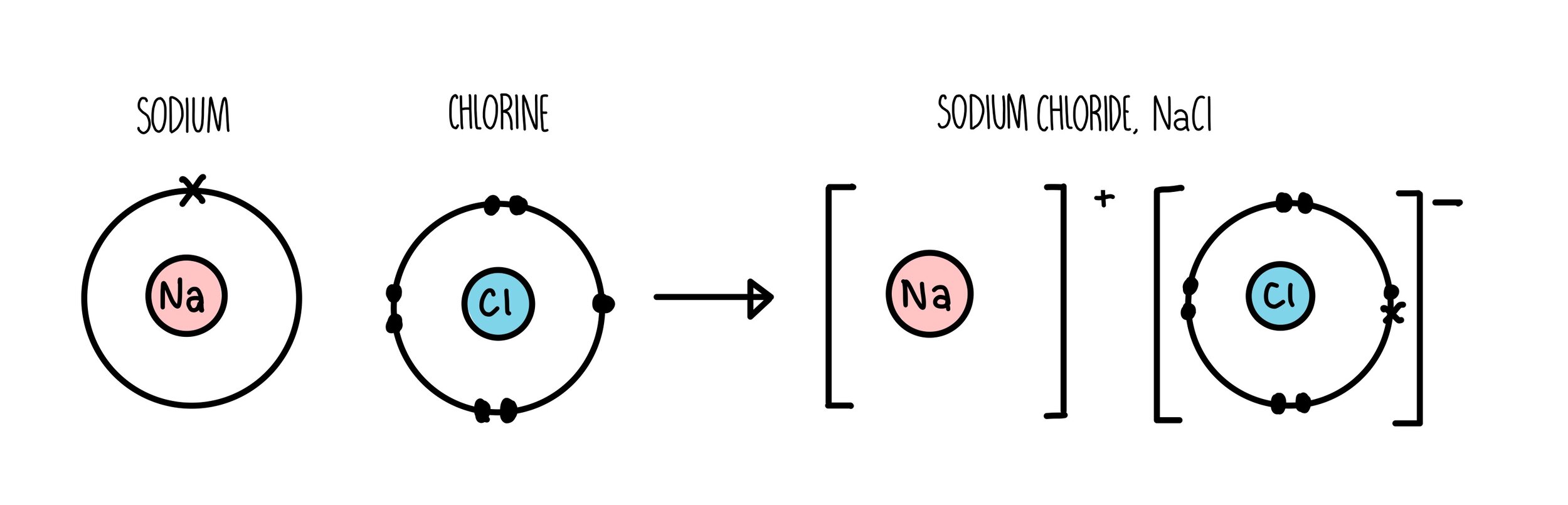
Ionic Bonding — the science hive
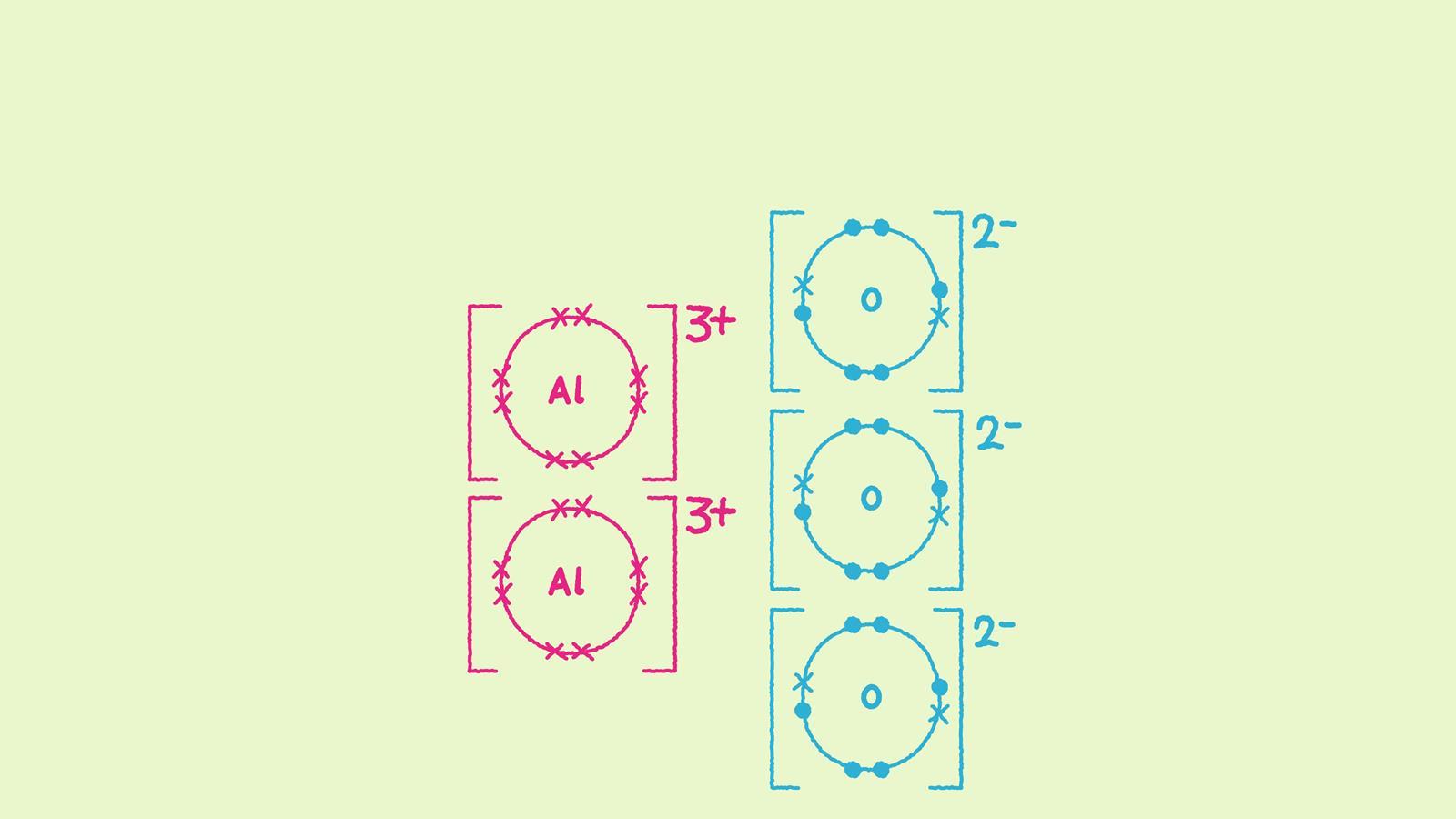
How to draw ionic bonding dot and cross diagrams Feature RSC Education

Ionic bonding practice

Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk
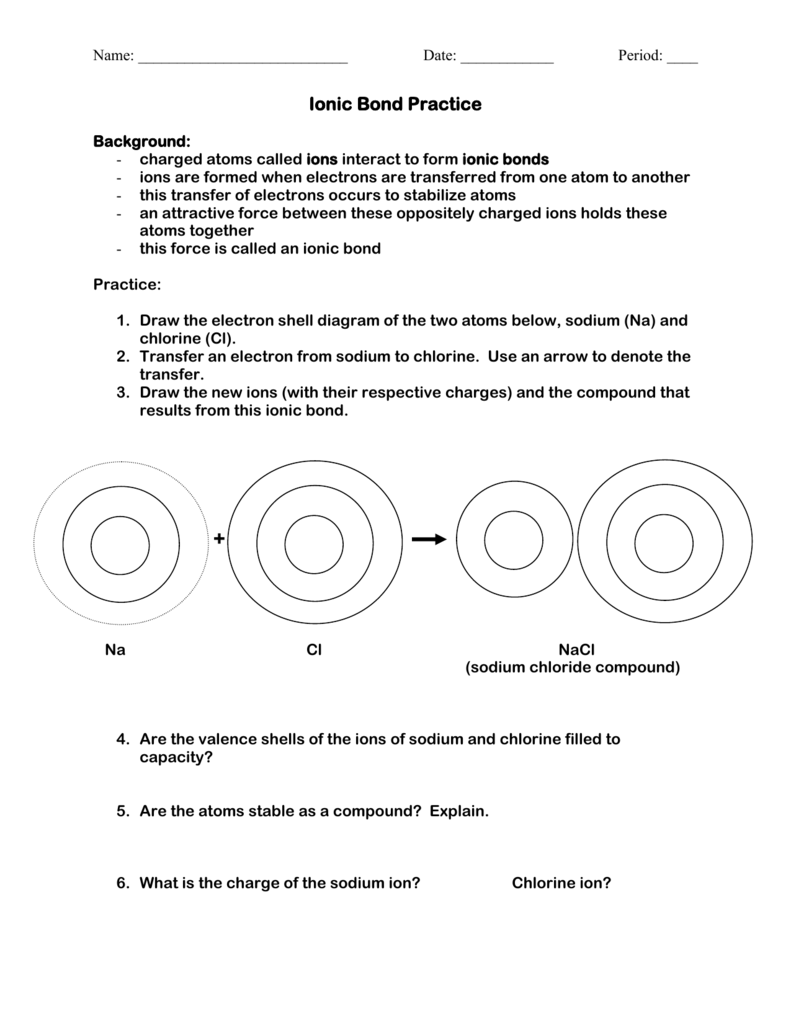
Ionic Bond Practice sciencewithskinner
Examples of Ionic Bonds and Compounds

Ionic And Covalent Bond Practice —

Ionic Bonds Practice worksheet Complete the chart for each element
Follow Your Teacher’s Directions To Complete Each Ionic Bond.
Cacl 2, Cucl 2, Cscl, Crcl 3;
Practice Drawing Lewis Dot Structures, Identifying.
Sicl 4, Pcl 3 Problem \(\Pageindex{3}\) For Each Of The Following Compounds, State Whether It Is Ionic Or Covalent.
Related Post:
