Nonmetals Drawing
Nonmetals Drawing - The main group metals are oxidized in all of their chemical reactions. These elements are distinctive in that they typically have low melting and boiling points, don't conduct heat or electricity very well, and tend to have high ionization energies and electronegativity values. These elements are often referred to as other nonmetals as the halogens and noble gases are also nonmetals. Most of the elements that comprise the human body—as well as the majority of other living things—are nonmetals. This is to verify that students And often poor conductors of heat and electricity. However, the elements either side of this border aren’t necessarily easy to classify. They generally very chemically reactive and are present in the environment as compounds rather than as pure elements. Example of some metals are. Physically, they are usually lighter (less dense) than metals; This section presents an overview of the properties and chemical behaviors of the nonmetals, as well as the chemistry of specific elements. Web updated on february 02, 2020. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. Web june 17, 2023 by jay rana. Nonmetals can be gases (such. Web (including the ones used in the previous sections of the lesson) and draw an x through the nonmetals. Metals are such element which have generally 1,2,3 valence electrons. Hydrogen acts as a nonmetal at normal temperatures and pressure and is generally accepted to be part of the nonmetal group. Web nonmetals are chemical elements that mostly lack distinctive metallic. And often poor conductors of heat and electricity. These elements are distinctive in that they typically have low melting and boiling points, don't conduct heat or electricity very well, and tend to have high ionization energies and electronegativity values. All three of them belong to the class of elements called nonmetals. Aluminum, for example, is oxidized by bromine. The main. Web one way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Their properties and behavior are quite different from those of metals on the left side. Where are nonmetals on the periodic table of elements? Web the term nonmetals is used to classify the elements h,. The line that divides metals from nonmetals in the periodic table crosses the p block diagonally. Web the metals, nonmetals, and metalloids concept builder is shown in the iframe below. They range from colorless gases like hydrogen to shiny crystals like iodine. These elements are distinctive in that they typically have low melting and boiling points, don't conduct heat or. Web the nonmetals are a group of elements in the periodic table. The halogens are nonmetals in group 7 of the periodic table. They include the most reactive and least reactive of elements, and they form many different ionic and covalent compounds. Elements in the same group share similar characteristics, like reactivity. The main group metals are oxidized in all. They comprise group 17 of the periodic table, from f through at. Their properties and behavior are quite different from those of metals on the left side. The halogen elements are a subset of the nonmetals. These are electronegative elements with high ionization energies. Web nonmetals are volatile, low melting and boiling points, low density, brittle or soft as solids,. Web one way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. They include the most reactive and least reactive of elements, and they form many different ionic and covalent compounds. These metals are oxidized when they react with nonmetal elements. This is to verify that students. Hydrogen acts as a nonmetal at normal temperatures and pressure and is generally accepted to be part of the nonmetal group. Physically, they are usually lighter (less dense) than metals; These metals are oxidized when they react with nonmetal elements. The halogens are nonmetals in group 7 of the periodic table. They generally very chemically reactive and are present in. Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. These nonmetals are located on the right side of the periodic table. Physically, they are usually lighter (less dense) than metals; The chemistry of the nonmetals is more interesting because these elements can. By brown,. Nonmetals on the periodic table are the elements that do not possess the metallic properties. Example of some metals are. There are 18 nonmetals on the periodic table. Web the metals, nonmetals, and metalloids concept builder is shown in the iframe below. This is to verify that students Their properties and behavior are quite different from those of metals on the left side. In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals. It works its way down between boron and aluminum, silicon, germanium, arsenic, antimony, and telirium. Web the border between metals and nonmetals is often drawn on the periodic table as a stepped line. These elements are often referred to as other nonmetals as the halogens and noble gases are also nonmetals. They include the most reactive and least reactive of elements, and they form many different ionic and covalent compounds. Web the term nonmetals is used to classify the elements h, c, n, p, o, s, and se. Web (including the ones used in the previous sections of the lesson) and draw an x through the nonmetals. Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. Web the nonmetals exhibit a rich variety of chemical behaviors. These are electronegative elements with high ionization energies.
Metals, nonmetals and metalloids

Ninth Metal Periodic Table 2023 Periodic Table Printable

Periodic Table Nonmetals Periodic Table Timeline
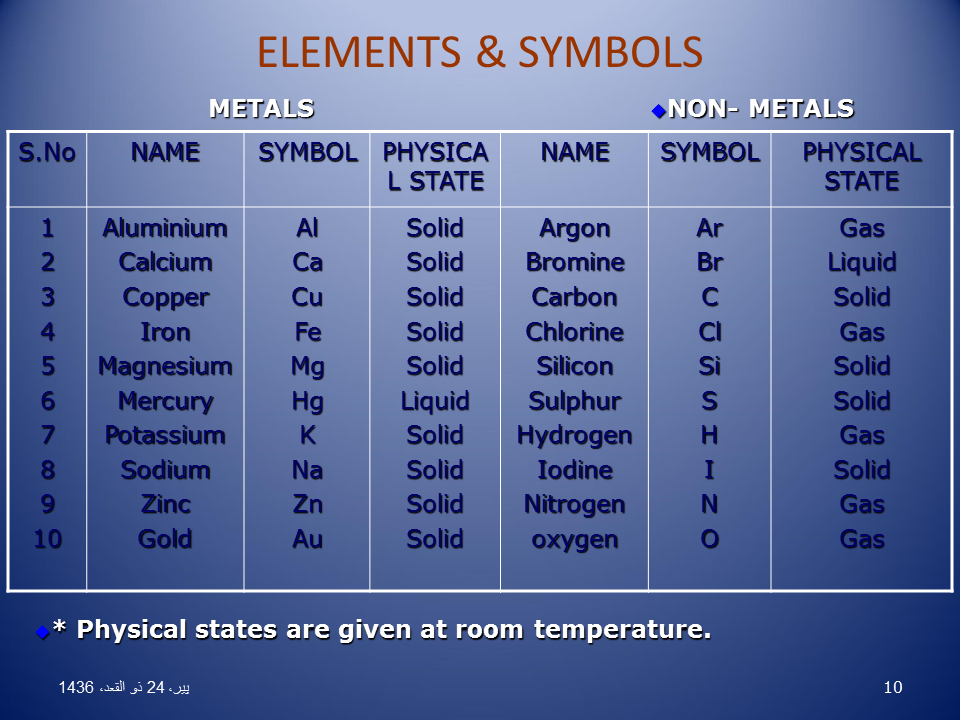
List of Non Metals with Symbols and Their Uses in Periodic Table

Nonmetals Knowledge Organiser Teaching Resources

Metals and Non Metals Video Properties and Uses What are metals and

Lesson Video Physical Properties of Metals and Nonmetals Nagwa
:max_bytes(150000):strip_icc()/metals-versusnonmetals-608809-v3-5b56348946e0fb0037001987.png)
The Difference Between Metals and Nonmetals

Nonmetal Elements Definition, Properties & Examples Video & Lesson
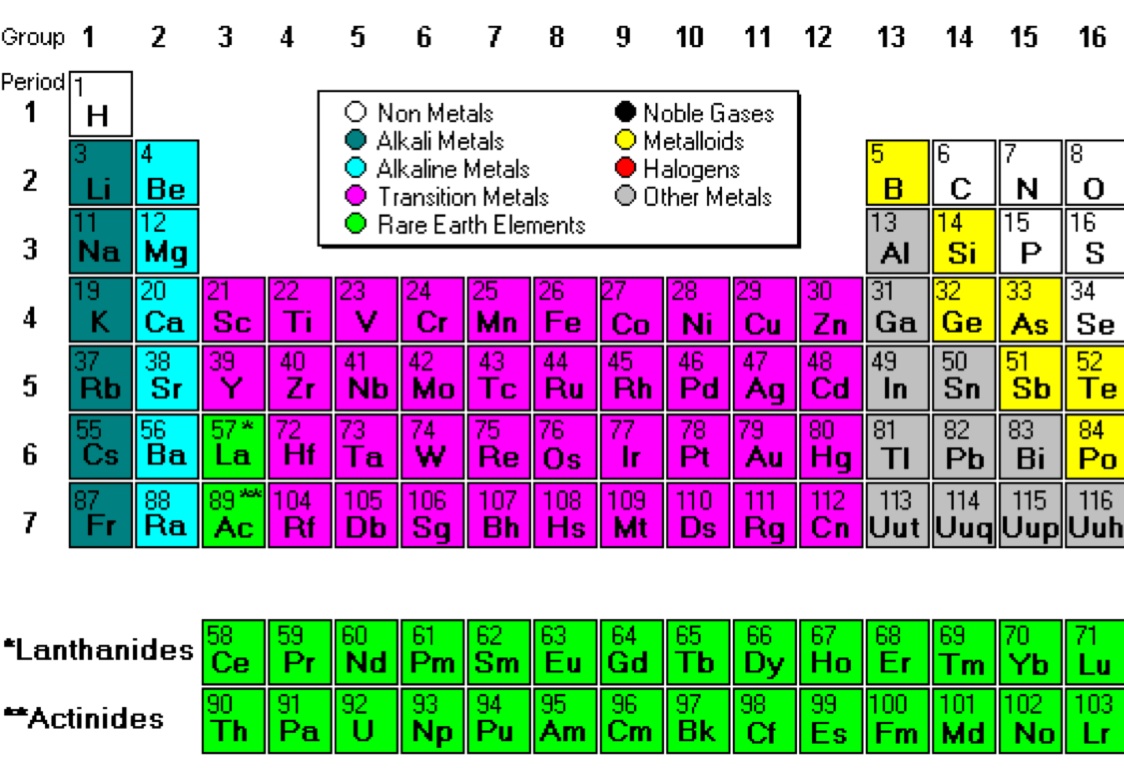
Periodic Table Of Elements Metals Nonmetals Metalloids Printable
The Halogen Elements Are A Subset Of The Nonmetals.
The Nonmetals Are Located On The Upper Right Side Of The Periodic Table.
Web Updated On February 02, 2020.
Web Nonmetals Are Volatile, Low Melting And Boiling Points, Low Density, Brittle Or Soft As Solids, Low Thermal And Electrical Conductance, Solids Dull In Appearance, Many Are Discrete Small Molecules With Atoms Joined By Strong Covalent Bonds, Intermolecular Forces Between Molecules Are Weak, Chemical Properties Characterized By Their Tendency To.
Related Post: