Lewis Structures How To Draw
Lewis Structures How To Draw - Web the organic chemistry tutor. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. Add electrons to form single bonds between the atoms. Ask me questions on facebook:. Write the 2 atomic symbols side by side. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. Fill the octets of the atoms bonded to the central atom. 2.1m views 6 years ago new ap & general chemistry video playlist. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Lewis structures of covalent compounds and ions. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Ask me questions on facebook:. Web how to draw lewis structures. This is a whiteboard animation tutorial on how to draw lewis structures of molecules. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. You figure out what the connections between atoms are. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. The diagram is also called a lewis dot. 439k views 5 years ago new organic chemistry playlist. Using lewis structures to show valence electrons. Web the albert team. Web the organic chemistry tutor. The valence electrons are the electrons in the. Web a video tutorial for how to draw lewis structures in five steps. The example is for the nitrate ion. In all cases, these bonds involve the sharing or transfer of. Web a lewis structure is a graphic representation of the electron distribution around atoms. A lewis electron dot formula comprises one dot for every valence electron and an element’s. Web there are several steps to follow when drawing lewis structures: It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. Web the organic chemistry tutor. The diagram is also called a lewis dot diagram, lewis dot. To use the lewis structure calculator follow these steps: The video covers the basic lewis structures you'll see in an introductor. The valence electrons are the electrons in the. Web here are the steps to draw a lewis structure. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Determine the central atom and connect the other atoms to it. Stages to articulate the electron dot formula are stated beneath. Fill the octets of the atoms bonded to the central atom. 2.1m views 6 years ago new ap & general chemistry video playlist. Thus far in this chapter, we have discussed the various types of bonds that form between. 698k views 13 years ago. Stages to articulate the electron dot formula are stated beneath. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. Using the periodic table to draw lewis dot structures. 439k views 5. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web here's how i draw lewis structures. Web a lewis structure is a graphic representation of the electron distribution around. Count the valence electrons for each atom, add them up, and add or remove electrons if there is an overall charge. Web a video tutorial for how to draw lewis structures in five steps. Web the organic chemistry tutor. Fill the octets of the atoms bonded to the central atom. A lewis structure is a structural representation of a molecule. How to draw electron dot structures? Web by bhattaru in workshop science. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. To use lewis dot symbols to explain the stoichiometry of a compound. 2.1m views 6 years ago new ap & general chemistry video playlist. Determine the total number of valence electrons in the molecule. Web how to draw lewis structures. / chemistnate how to draw lewis structures (aka lewis dot diagrams).more. A lewis structure is a structural representation of a molecule where dots are used to show electron position around the atoms. Fill the octets of the atoms bonded to the central atom. This organic chemistry video tutorial explains how to draw lewis. Determine the central atom and connect the other atoms to it. Lewis structures show all of the valence electrons in an atom or molecule. A lewis structure also helps to make a prediction about the geometry of a molecule. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Lewis structure, also known as lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule.
3 Ways to Draw Lewis Dot Structures wikiHow
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure

How To Draw Lewis Structures A Comprehensive Guide IHSANPEDIA

How to Draw a Lewis Structure

Molecular Modeling Digital and Analog Middlebury College Chem 103 lab
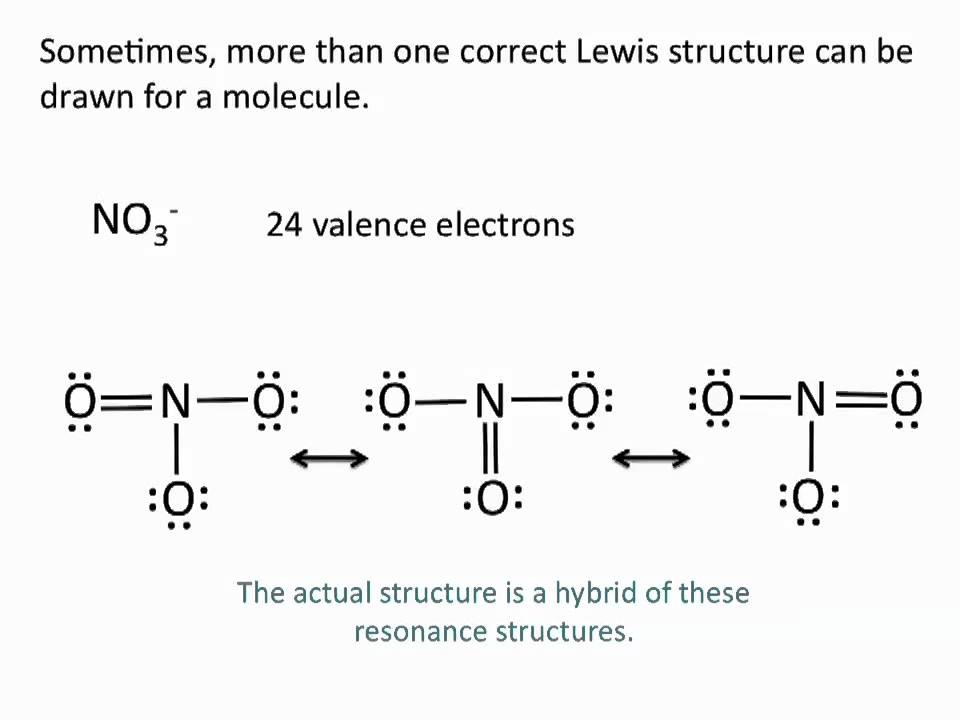
Drawing Lewis Structures Resonance Structures Chemistry Tutorial

Lewis Diagrams Made Easy How to Draw Lewis Dot Structures Watch

How to Draw a Lewis Structure

3 Ways to Draw Lewis Dot Structures wikiHow

How to draw Lewis Structures a step by step tutorial Middle School
It Will Also Work With More Complex Molecules And Ions, If You Recognize That Individual Atoms Will Have The Same Arrangement Of Bonds And Lone Pairs As They Do In The Simple Structures.
Shared Pairs Of Electrons Are Drawn As Lines Between Atoms, While Lone Pairs Of Electrons Are Drawn As Dots Next To Atoms.
Count The Valence Electrons For Each Atom, Add Them Up, And Add Or Remove Electrons If There Is An Overall Charge.
Lewis Structures Of Covalent Compounds And Ions.
Related Post: