How To Draw The Electron Configuration
How To Draw The Electron Configuration - The alkali metal sodium (atomic number 11) has one more electron than the neon atom. The rules above allow one to write the electron configurations for all the elements in the periodic table. 133k views 9 years ago. A simple tutorial on how to draw electron. In this tutorial, you will learn how to find and write the electron configurations and orbital diagrams for various elements using the periodic table. Identify and explain exceptions to predicted electron configurations for atoms and ions. Electrons must occupy the lowest available shell, closest to the nucleus. Shells are the allowed energy levels of electrons. We construct the periodic table by following the aufbau principle (from german, meaning “building up”). Web © 2024 google llc. Web use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. How to draw and write electronic structures (also called the electron configurations).an essential. 1.1m views 9 years ago chemistry: Web give the electron configuration for an atom using bohr’s model, box orbital diagrams, and quantum mechanical notation. Here are a few highlights. Shells are the allowed energy levels of electrons. How to draw and write electronic structures (also called the electron configurations).an essential. Web there's another way to write an electron configuration, or to draw one out, it's called orbital notation. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. In this tutorial, you will. Web use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. 1.1m views 9 years ago chemistry: Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. We're talking about an s orbital in the first. The rules above allow one to write the electron configurations for all the elements in the periodic table. Shells are the allowed energy levels of electrons. Web use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. How to draw and write electronic structures (also called the electron configurations).an essential. There is a. A simple tutorial on how to draw electron. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. 77 views 8 months ago c1 atomic structure. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. 133k views 9. Web the electron configurations and orbital diagrams of these four elements are: The total number of electrons is the atomic number, z. This video also includes ques. Web give the electron configuration for an atom using bohr’s model, box orbital diagrams, and quantum mechanical notation. Web © 2024 google llc. Web the electron configurations and orbital diagrams of these four elements are: There is a specific notation that can quickly show you where the electrons are likely to be located, so knowing this notation is an essential part of knowing electron configurations. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. Web. Web © 2024 google llc. The rules above allow one to write the electron configurations for all the elements in the periodic table. The first shell has the lowest energy and the energies increase as the electrons get further away from the positively charged nucleus. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons. It contains plenty of practice problems including the electron configuration of transition metal ions. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web the electron configurations and orbital diagrams of these four elements are: Shells are the allowed energy levels of electrons. You will learn aufbau’s. Web electron configurations describe where electrons are located around the nucleus of an atom. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. You will learn aufbau’s principle, hund’s rule. It explains how to write the orbital diagram n. By knowing the. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for. How to draw and write electronic structures (also called the electron configurations).an essential. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. The total number of electrons is the atomic number, z. So you draw a line here, which represents an orbital. You will learn aufbau’s principle, hund’s rule. Two in the first shell, eight in the second shell, eight in the third shell. In this tutorial, you will learn how to find and write the electron configurations and orbital diagrams for various elements using the periodic table. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. This video also includes ques. 1.1m views 9 years ago chemistry: For example, the electron configuration of sodium is 1s 2 2s 2 2p 6 3s 1. The rules above allow one to write the electron configurations for all the elements in the periodic table. Here are a few highlights that you learned in the last section about bohr's model: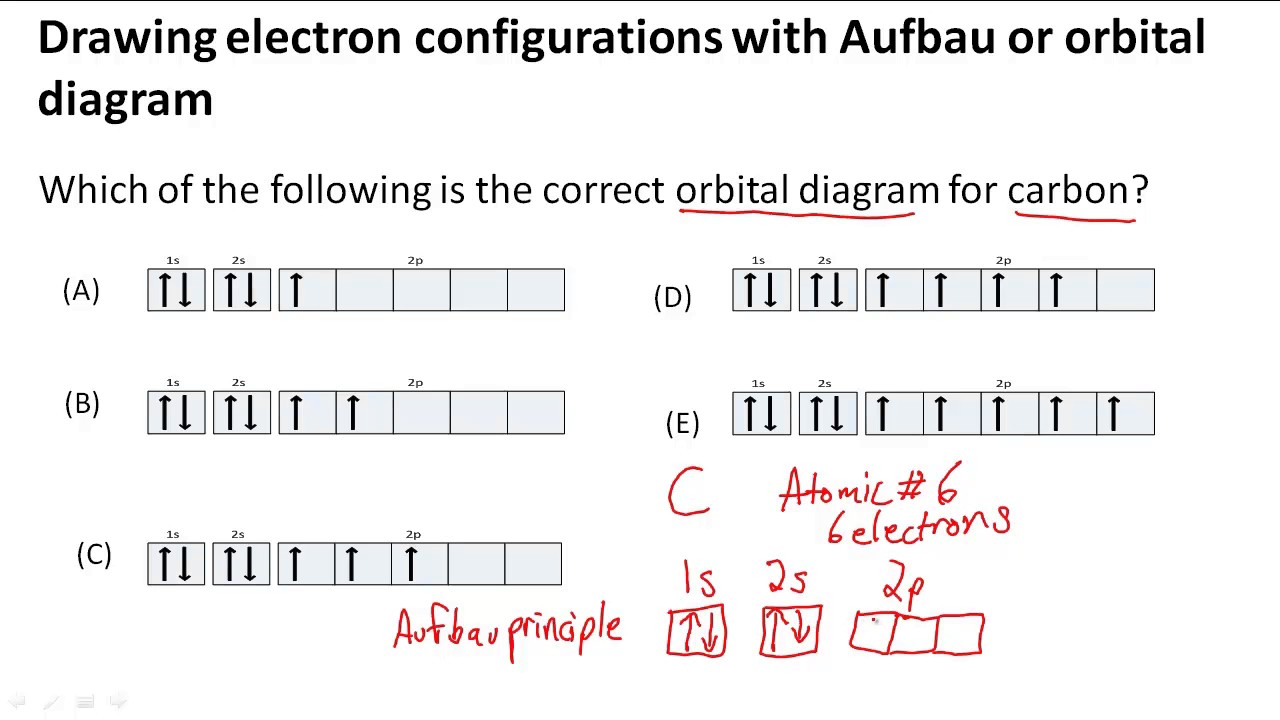
Drawing electron configurations with Aufbau/orbital diagram YouTube
Drawing electron configuration diagrams Chemistry for All The Fuse
Drawing electron configuration diagrams YouTube
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Electron Configuration Chart

ALevel Chemistry 1.6.1c draw electron configuration diagrams of
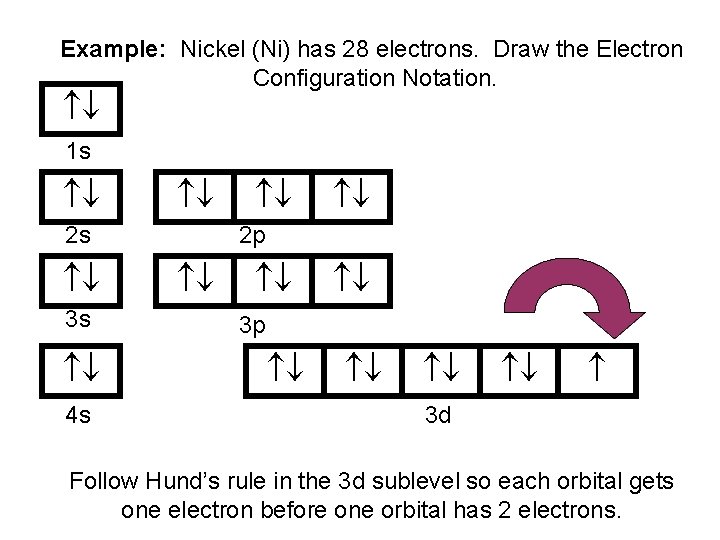
Electron Configuration Chapter 5 Electrons have 3 levels
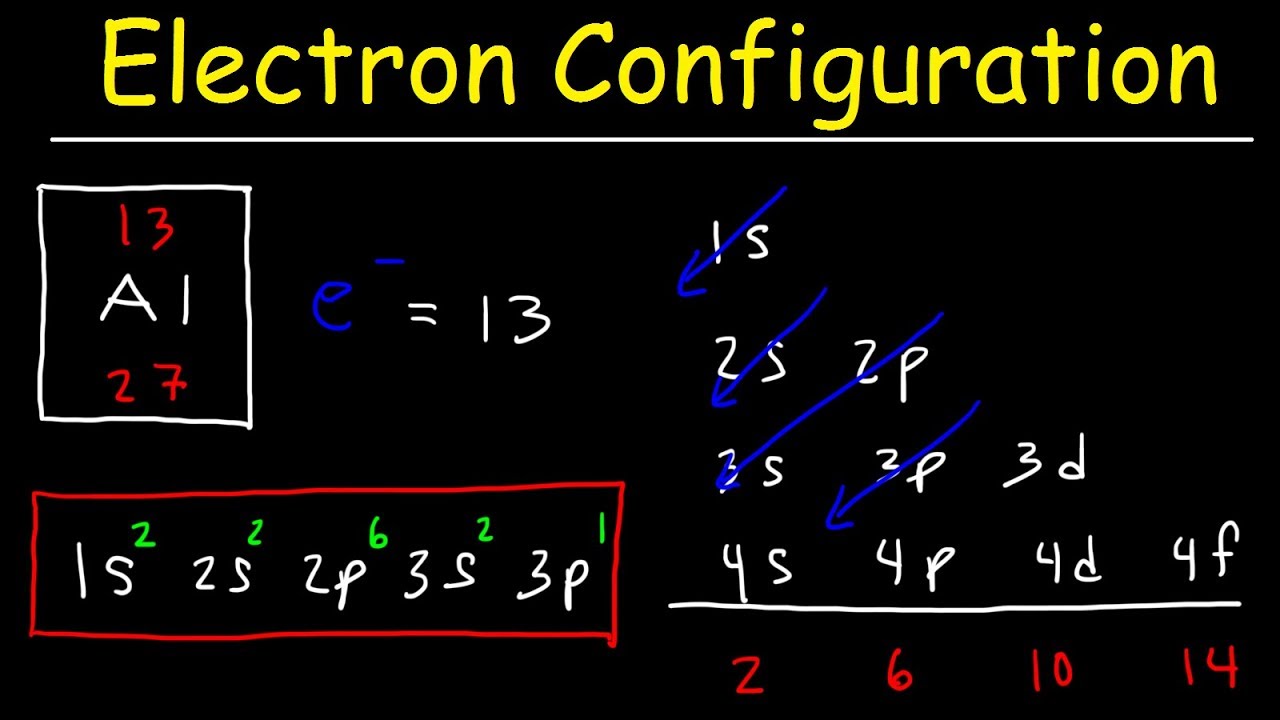
Electron Configuration Basic introduction YoutuBeRandom
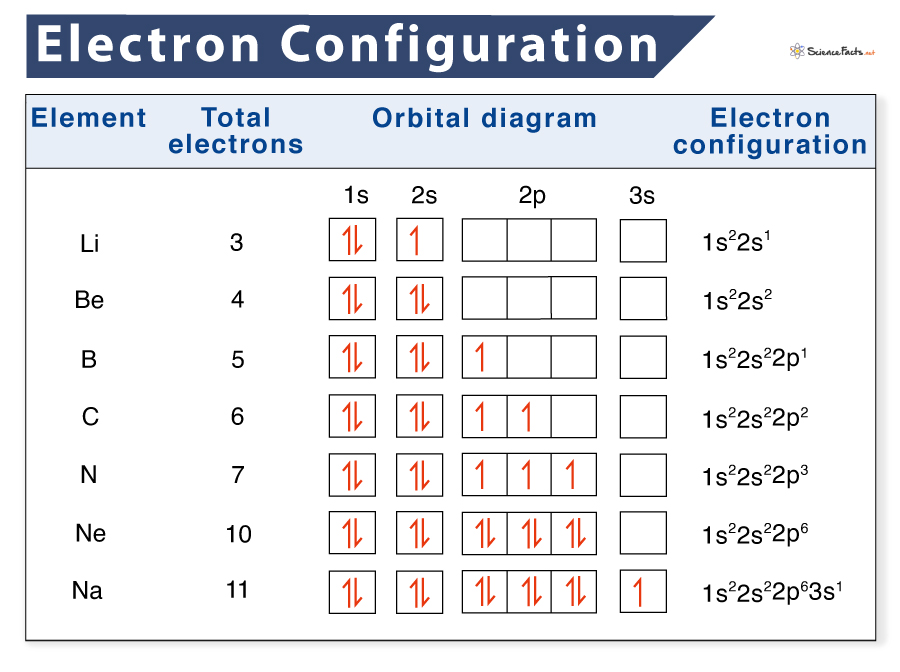
Electron Configuration Definition, Examples, Chart, and Diagram

2.2 Electron Configurations Chemistry LibreTexts

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts
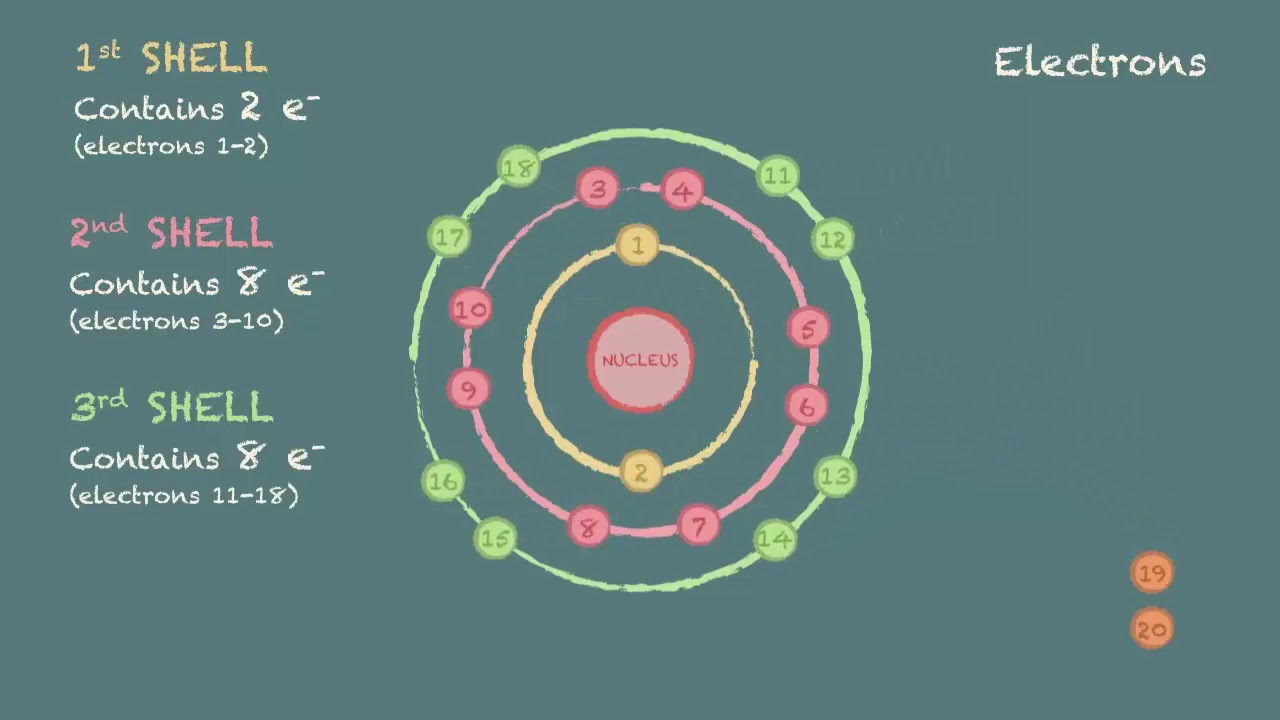
This Is Sometimes Called The Bohr, Or The ‘Solar System’, Model.
Web Chemistry 1.1 (Gmit) 5:
Web Revise Chemistry With Mr B.
Shared Pairs Of Electrons Are Drawn As Lines Between Atoms, While Lone Pairs Of Electrons Are Drawn As Dots Next To Atoms.
Related Post: