How To Draw The Atomic Structure
How To Draw The Atomic Structure - Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Rutherford’s gold foil experiment (opens a modal) Relate electron configurations to element classifications in. While atoms of hydrogen, nitrogen and oxygen do not exist independently. For example, he and ne, etc. To indicate they are protons, draw them as circles with plus signs contained inside. The structure of the atom. A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. The negatively charged particles called electrons revolve around the centre of the nucleus. Erase the c in the center circle, and draw in your protons. (hydrogen is excluded because it can hold a. The structure of the atom. Have atoms, which exists independently. Web in this video i have used the example of sodium, simply because it has been the first question on every chemistry paper that i have done :) Web student note:this video will show you how to draw atoms of the first. After completing this section, you should be able to. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight valence electrons (an octet). For example, he and ne, etc. Then play a game to test your ideas! Since protons are. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight valence electrons (an octet). Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Build an atom out of protons, neutrons, and electrons,. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Let's learn. Let's learn how to draw an atomic. You can also play a fun game to check your understanding of atomic concepts. 1.9m views 9 years ago chemistry learning sequence. The negatively charged particles called electrons revolve around the centre of the nucleus. Rutherford’s gold foil experiment (opens a modal) Relate electron configurations to element classifications in. Web chemspider is a free online database of chemical structures and properties. (hydrogen is excluded because it can hold a. To indicate they are protons, draw them as circles with plus signs contained inside. Neutrons are simply equal to the atomic mass minus the number of protons. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Follow us at / atomicschool , / atomicschools and /. The structure of the atom. Write the molecular formula of. Let's learn how to draw an atomic. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Web draw your protons and neutrons. A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons. The atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons. According to dalton’s theory atom is smallest particle which could not be divided any further. Relate electron configurations to element classifications in. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they are surrounded by eight valence electrons (an octet). Chemspider also provides access. Web shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. This is atomic structure tutorial video on protons, electrons, and neutrons. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share electrons until they. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Neutrons are simply equal to the atomic mass minus the number of protons. Since protons are the same as the amount of electrons, you just draw 6 protons. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number. 49k views 6 years ago diagrams | diagram drawing. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. 1.9m views 9 years ago chemistry learning sequence. While atoms of hydrogen, nitrogen and oxygen do not exist independently. Web we recommend using the latest version of chrome, firefox, safari, or edge. The numbers of subatomic particles in an atom can be calculated from its. You can also play a fun game to check your understanding of atomic concepts. Web shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Erase the c in the center circle, and draw in your protons. Identify and explain exceptions to predicted electron configurations for atoms and ions. Relate electron configurations to element classifications in. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
Atom Drawing
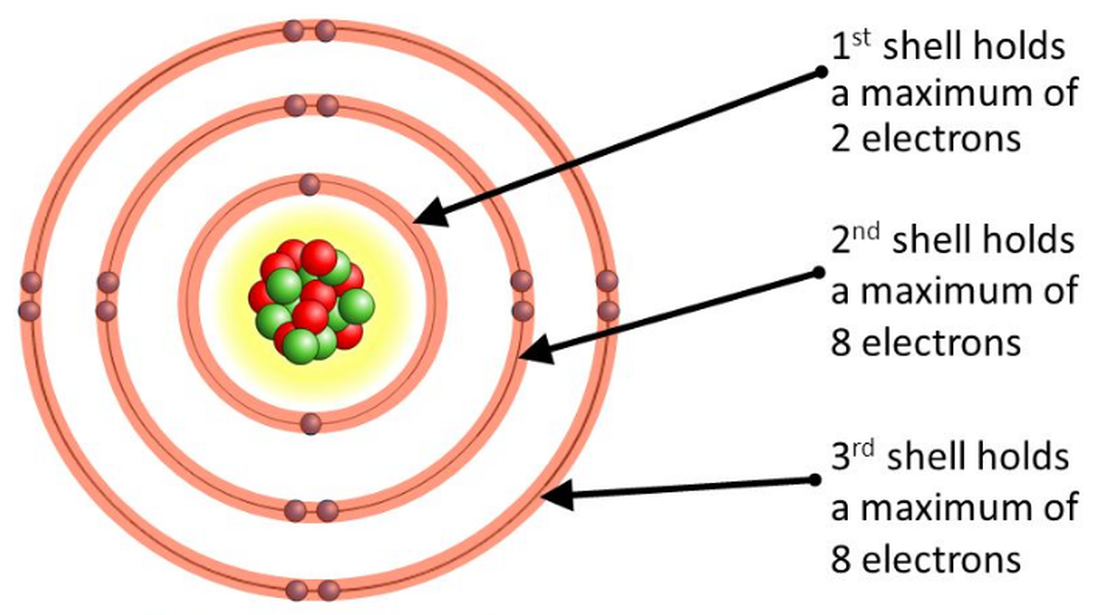
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons

How To Draw An Atomic Model Artistrestaurant2
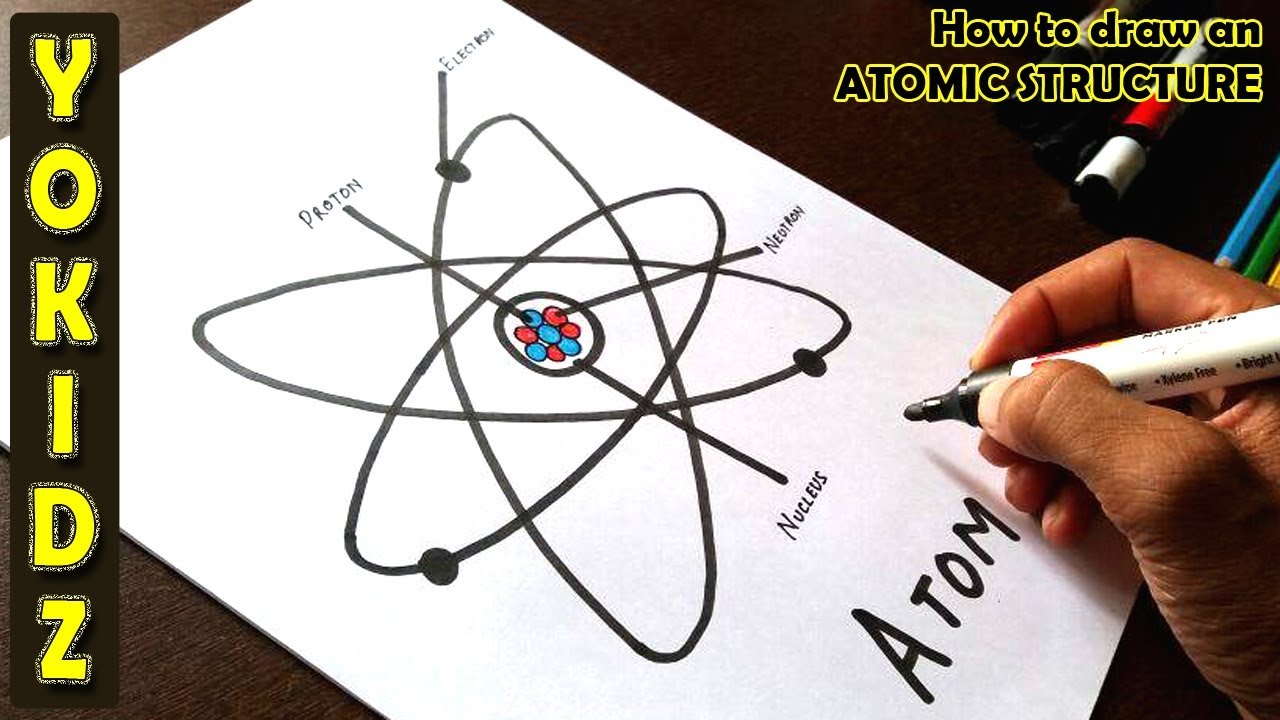
How to draw an ATOMIC structure YouTube

Atom Definition, Structure & Parts with Labeled Diagram

Atomic structure Learning Lab

Drawing Atoms Montessori Muddle
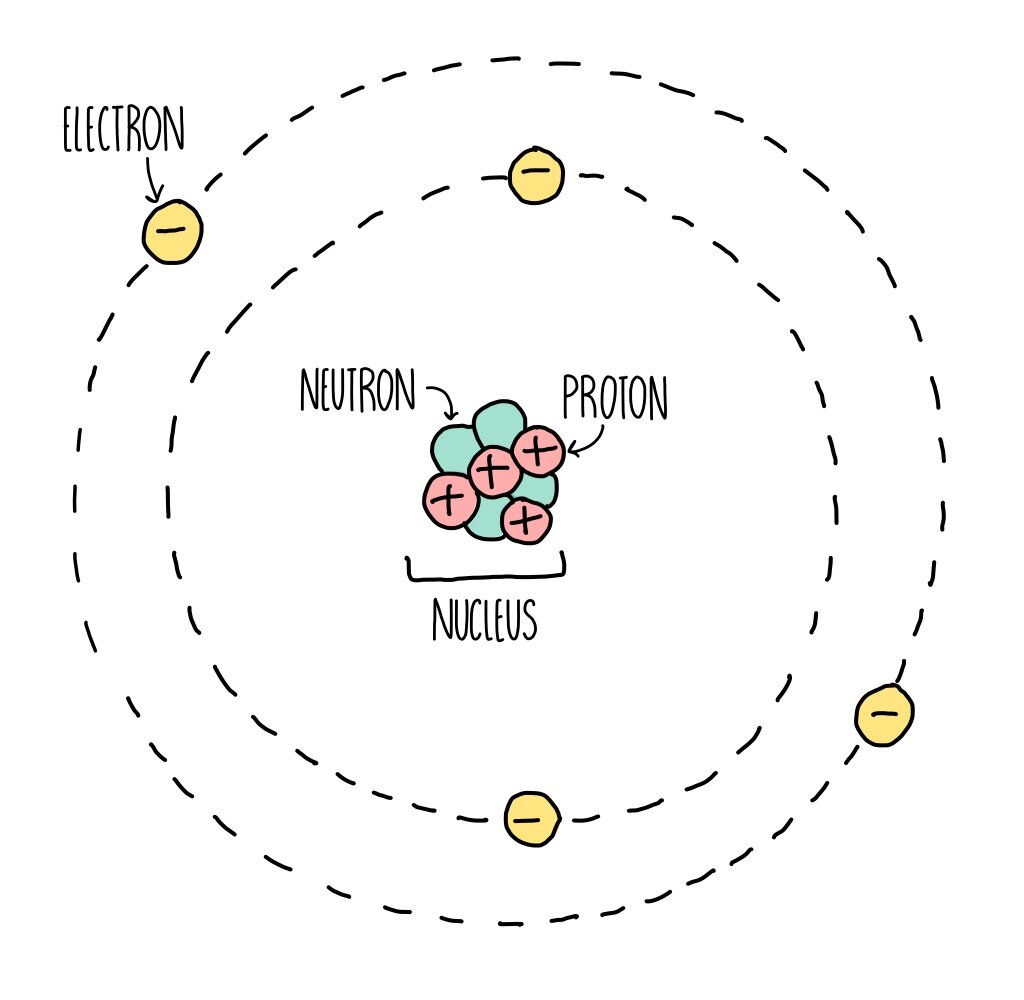
Atomic Structure (GCSE) — the science sauce

Draw the atomic structure of a chlorine ion Brainly.in

How to draw Atom structure diagram step by step l Atomic structure
Rutherford’s Gold Foil Experiment (Opens A Modal)
Web Electron Configurations And The Periodic Table.
Try This Interactive Simulation And Explore The Structure And Symbols Of Atoms, Isotopes, And Ions.
A Rule Stating That Atoms Lose, Gain, Or Share Electrons In Order To Have A Full Valence Shell Of 8 Electrons.
Related Post: