How To Draw Lewis Structures For Ions
How To Draw Lewis Structures For Ions - Valence electrons are the electrons present in the outermost shell of the electronic configuration of an atom. This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as. Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. Web define ionic bond. Valence electrons of nitrogen atom and chlorine atom. Web to use the lewis structure calculator follow these steps: The number of valence electrons in an individual atom can be found based on the atom’s group number in the periodic table. Covalent bonds are formed when one electron from each atom forms an electron pair. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. Then we write the rest of the formula. Covalent bonds are formed when one electron from each atom forms an electron pair. If you haven’t mastered the art of drawing lewis structures yet, you can check out the tutorial by clicking here. Whether you are a student, a teacher, or a chemistry enthusiast, you will find this webpage helpful and. Web the organic chemistry tutor. It will also. Determine the number of bonds in the molecule. The astute reader may have noticed something: Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. To show this, draw brackets around the atomic (or polyatomic) symbol. Web here are the steps to. Enter the formula of the molecule in the field provided for it. 126k views 3 years ago new ap & general chemistry video playlist. In all cases, these bonds involve the sharing or transfer of. The example is for the nitrate ion. Count the total valence electrons. 292k views 3 years ago new ap & general chemistry video playlist. 65k views 5 years ago. In all cases, these bonds involve the sharing or transfer of. Then we write the rest of the formula. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. Then we write the rest of the formula. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. In all cases, these bonds involve the sharing or transfer of. This chemistry video explains how to draw the lewis structures of ionic compounds. Web the organic chemistry tutor. Web here are the steps to draw a lewis structure. 292k views 3 years ago new ap & general chemistry video playlist. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. Lewis. 292k views 3 years ago new ap & general chemistry video playlist. Web there are several steps to follow when drawing lewis structures: A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. Web do you want to learn how to draw lewis dot structures for polyatomic ions in a clear and. Web do you want to learn how to draw lewis dot structures for polyatomic ions in a clear and simple way? Web for very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. In all cases, these bonds involve the sharing or transfer of. Web lewis structures. Web to use the lewis structure calculator follow these steps: Web designate the charge of the ion. Counting dots on every atom would be a tedious way of determining if that atom had a charge. The following is a general procedure for drawing lewis structures. Web the organic chemistry tutor. If you haven’t mastered the art of drawing lewis structures yet, you can check out the tutorial by clicking here. Web do you want to learn how to draw lewis dot structures for polyatomic ions in a clear and simple way? Web how to draw lewis structures. The diagram is also called a lewis dot diagram, lewis dot formula, or. 65k views 5 years ago. Draw lewis structures for ionic compounds. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Web how to draw lewis structures. Covalent bonds are formed when one electron from each atom forms an electron pair. Counting dots on every atom would be a tedious way of determining if that atom had a charge. The following is a general procedure for drawing lewis structures. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. The astute reader may have noticed something: If you haven’t mastered the art of drawing lewis structures yet, you can check out the tutorial by clicking here. 292k views 3 years ago new ap & general chemistry video playlist. Web draw lewis structures depicting the bonding in simple molecules. To make the structures easier to read, you need to show that your structure is an ion with some charge. Web there are several steps to follow when drawing lewis structures: The example is for the nitrate ion.
3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Dot Structures of Atoms and Ions YouTube

Lewis Diagrams Made Easy How to Draw Lewis Dot Structures Watch
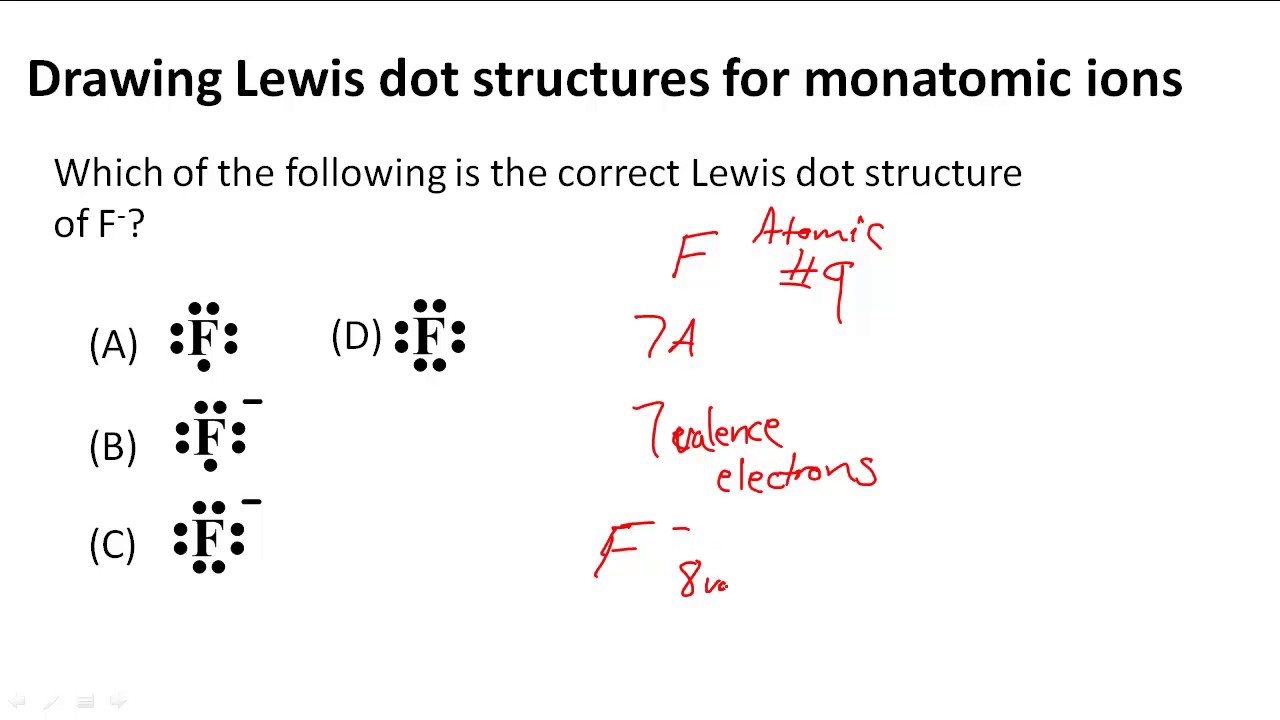
Drawing Lewis dot structures for monatomic ions YouTube

Nitrate Ion Lewis Structure How to Draw the Lewis Structure for

Lewis Dot Structure Definition, Examples, and Drawing

How to Draw a Lewis Structure
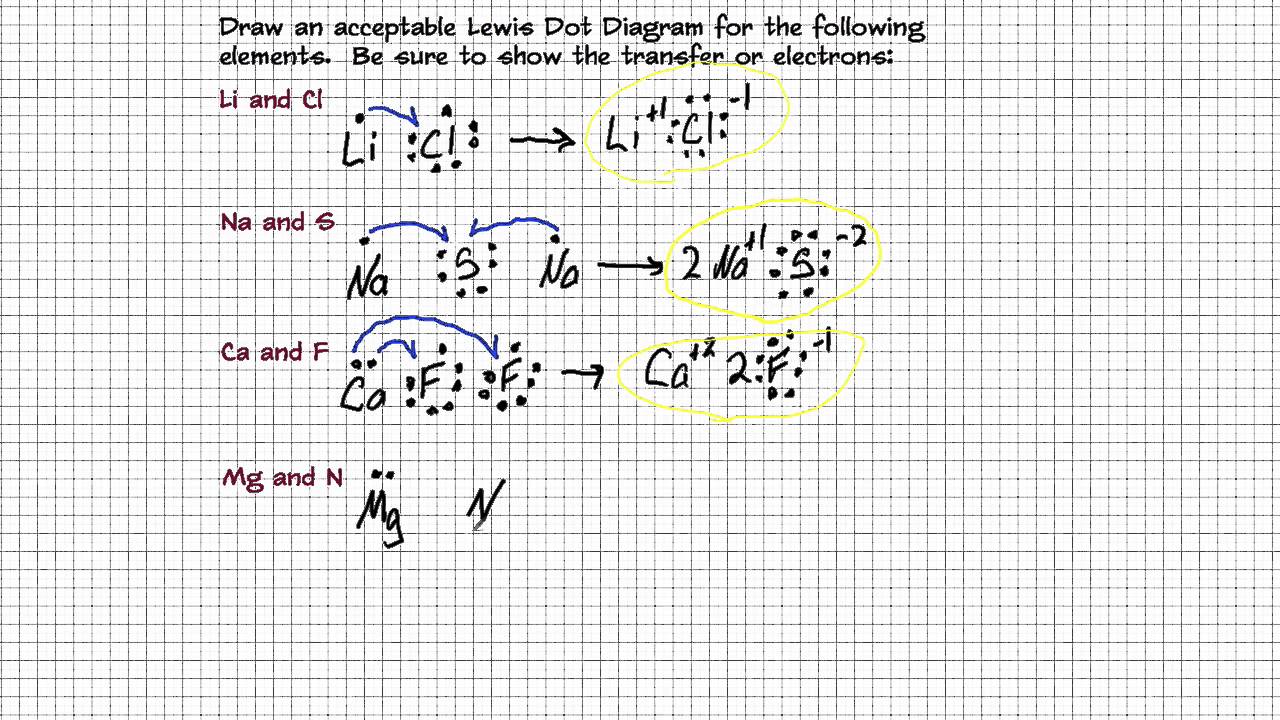
Drawing Lewis Dot Diagrams for Ionic Compounds YouTube
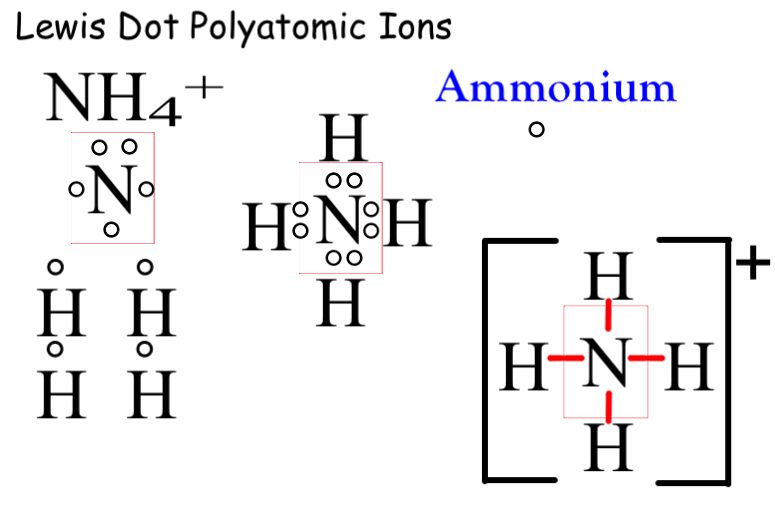
How do you draw lewis structures for polyatomic ions? Socratic

OH Lewis Structure How to Draw the Lewis Dot Structure for the
Add Electrons To Form Single Bonds Between The Atoms.
The Valence Electrons Are The Electrons In The.
126K Views 3 Years Ago New Ap & General Chemistry Video Playlist.
Web To Draw The Lewis Structure, You Will Need To Know The Total Number Of Valence Electrons Present.
Related Post: