How To Draw Lewis Dot Structures For Single Elements
How To Draw Lewis Dot Structures For Single Elements - In all cases, these bonds involve the sharing or transfer of. The ch2o c h 2 o molecule. Web drawing lewis dot structures. Oxygen satisfies the octet rule as the most electronegative and carbon the least electronegative between the two and hence we see the electron. To instruct the student on how to draw the lewis dot structures for simple, covalent compounds. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Web how to draw lewis structures. Web draw lewis structures depicting the bonding in simple molecules. Web here are the steps to draw a lewis structure. How do you draw the lewis structure for ions? Web draw lewis structures depicting the bonding in simple molecules. Write the atomic symbol for each atom. By the end of this section, you will be able to: What are some examples of lewis structures? Web to draw the lewis structure, you will need to know the total number of valence electrons present. The h2o h 2 o molecule. Web to draw the lewis structure, you will need to know the total number of valence electrons present. The number of dots equals. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Determine the number of bonds in the molecule. What is the lewis structure for so2? Web the easy method procedure to determine a lewis dot structure. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. Web drawing lewis dot structures. What is the lewis structure for so2? 1) determine which atoms are connected to each other. To instruct the student on how to draw the lewis dot structures for simple, covalent compounds. Web drawing lewis dot structures. Below is a table that coordinates the group number to the valence electrons. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. 3) place two electrons between each atom in place of the bonds. The example is for the nitrate ion. Be sure to have the correct number of electrons. Determine the total number of valence electrons for the formula. The number of valence electrons in an individual atom can be found based on the atom’s group number in the periodic table. The following is a general procedure for drawing lewis structures. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they. To use lewis dot symbols to explain the stoichiometry of a compound. These symbols will represent the atoms present in the covalent bond. By the end of this section, you will be able to: Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Web lewis structures (also known as lewis dot structures. Determine the number of bonds in the molecule. Web draw lewis structures depicting the bonding in simple molecules. The ocl− o c l − ion. By the end of this section, you will be able to: The example is for the nitrate ion. Web lewis structures are drawn by a series of dots, lines, and atomic symbols and provide a structure for the way that the atom or molecule is arranged. A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. Oxygen satisfies the octet rule as the most electronegative and carbon the least electronegative. Draw a lewis electron dot diagram for an atom or a monatomic ion. What are some examples of lewis structures? It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. To facilitate our understanding of how valence. These lewis symbols and lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. The number of valence electrons in an individual atom can be found based on the atom’s group number in the periodic table. The h2o h 2 o molecule. What are lewis dot structures used for? The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Subtracting the number in step 1 from the number in step 2 gives you the number of electrons needed to. To instruct the student on how to draw the lewis dot structures for simple, covalent compounds. Below is a table that coordinates the group number to the valence electrons. It will also work with more complex molecules and ions, if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. Be sure to leave enough space between the atoms to draw your electrons and bonds. How do you draw the lewis structure for ions? A lewis dot structure can be made for a single atom, a covalent compound, or a polyatomic ion. Web steps to drawing lewis dot structures: A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. If the species is an ion, add or subtract electrons corresponding to the charge.
3 Ways to Draw Lewis Dot Structures wikiHow
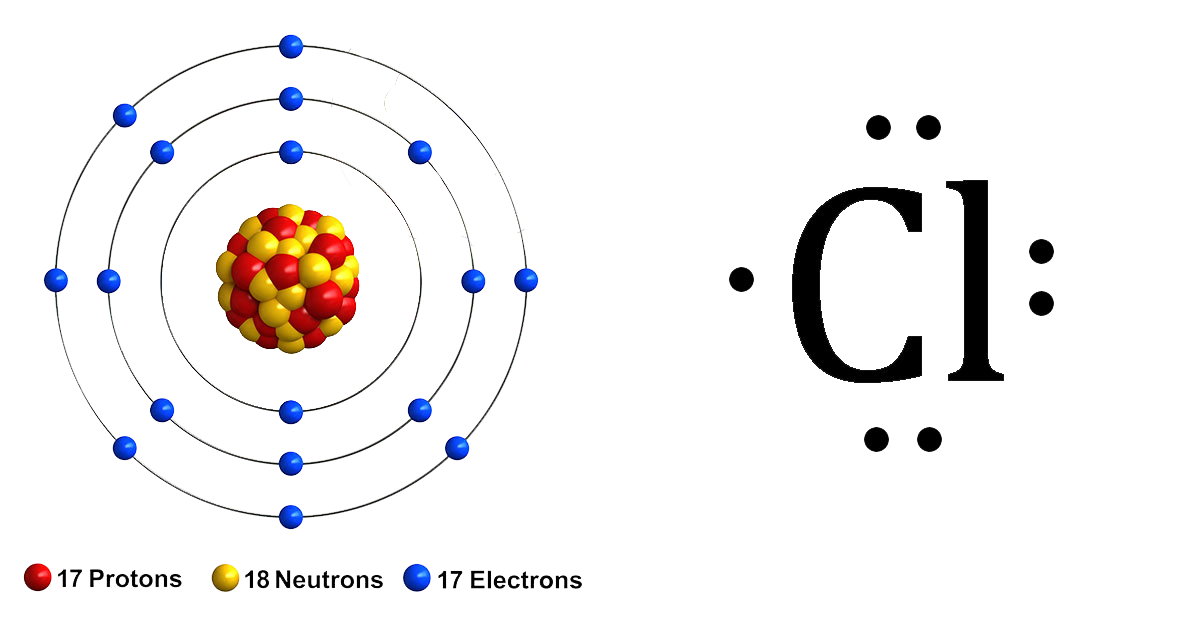
How To Draw Lewis Dot Diagrams Simplereality27
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
Best How To Draw The Lewis Dot Structure in the year 2023 The ultimate

How to draw Lewis Structures a step by step tutorial Middle School
How To Draw Lewis Dot Diagrams Simplereality27

How to Draw a Lewis Structure

Single atom lewis dot structures YouTube

Drawing Lewis Dot Structures for Chemistry dummies
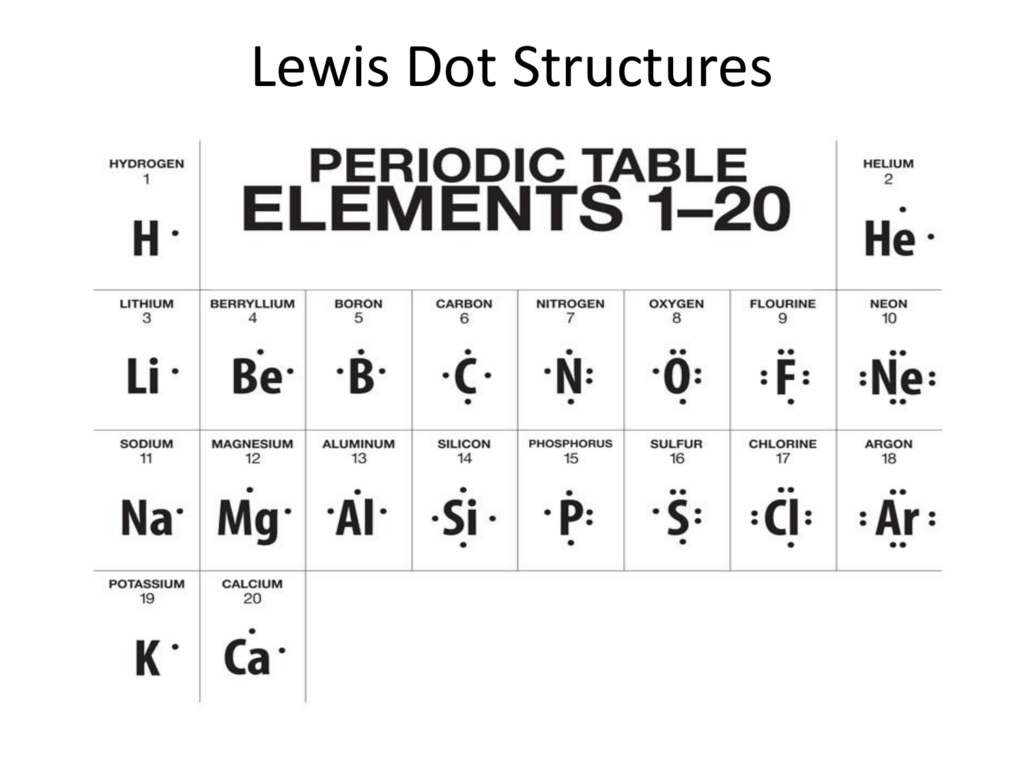
Lewis Dot Structures

Lewis Dot Structure Definition, Examples, and Drawing
Web To Draw The Lewis Structure, You Will Need To Know The Total Number Of Valence Electrons Present.
Follow These Simple Steps To Draw Lewis Dot Structures:
The Element Symbol, Together With The Dots, Is Called The Lewis Symbol.
Draw The Atoms On Paper And Put Dots Around Them To Represent Valence Electrons Of The Atom.
Related Post: