How To Draw Lewis Dot Structures For Ionic Compounds
How To Draw Lewis Dot Structures For Ionic Compounds - You can see a listing of all my. What is a lewis dot structure? Explain the formation of ions as an atom transfers electrons to another atom. Using the periodic table to draw lewis dot structures. A lewis structure is named after gilbert lewis, who. The valence electrons are the electrons in the. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. The astute reader may have noticed something: Web ionic lewis dot structures. The example is for the nitrate ion. The example is for the nitrate ion. Remember that lewis dot structures. Shows how to draw lewis dot structures for ionic compounds. What is an example of a lewis structures practice problem? Then we write the rest of the formula. Web lewis structures are mostly applied to covalent molecules, and while it is exceedingly uncommon you should do the same for ionic compounds. Stages to articulate the electron dot formula are stated beneath. Determine the total number of valence electrons in the molecule or ion. What is an example of a lewis structures practice problem? Lewis structures show all of. Molecules with covalent bonds and compounds held together by ions can have lewis structures. Atoms & elements 4h 15m. The valence electrons are the electrons in the. How to draw lewis dot structures. 166k views 11 years ago chemical equations; You can see a listing of all my. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Remember that lewis dot structures. Then we write the rest of the formula. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web how do you draw the lewis structure for ionic compounds? How to draw a lewis structure. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. The two ions attract each other according to coulombic interactions. Lewis structures show all of the valence electrons in an atom or molecule. 1.1 what does a lewis dot structure represent? In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Instead ionic compounds stick together through electrostatic forces (different electrically. Determine the total number of valence electrons in the molecule or ion. A lewis electron dot formula comprises one dot. How to draw a lewis structure. Chemistry covalent bonds drawing lewis structures. Molecules with covalent bonds and compounds held together by ions can have lewis structures. When you draw an ion, don't forget [ ] and a charge. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element. Web lewis structures are mostly applied to covalent molecules, and while it is exceedingly uncommon you should do the same for ionic compounds. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Explain the formation of ions. Chemistry covalent bonds drawing lewis structures. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. How to draw a lewis structure. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. The valence electrons are the electrons in. 166k views 11 years ago chemical equations; When drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. In all cases, these bonds involve the sharing or transfer of. Covalent bonds work. The example is for the nitrate ion. Instead ionic compounds stick together through electrostatic forces (different electrically. The astute reader may have noticed something: What is an example of a lewis structures practice problem? Using the periodic table to draw lewis dot structures. When you draw an ion, don't forget [ ] and a charge. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, 3.1 lewis structure of a cation. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Web how do you draw the lewis structure for ionic compounds? The two ions attract each other according to coulombic interactions. Web the albert team. When drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for covalent/molecular. How to draw lewis dot structure? Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. A lewis structure is a way to show how atoms share electrons when they form a molecule.
Lewis Dot Structure Definition, Examples, and Drawing
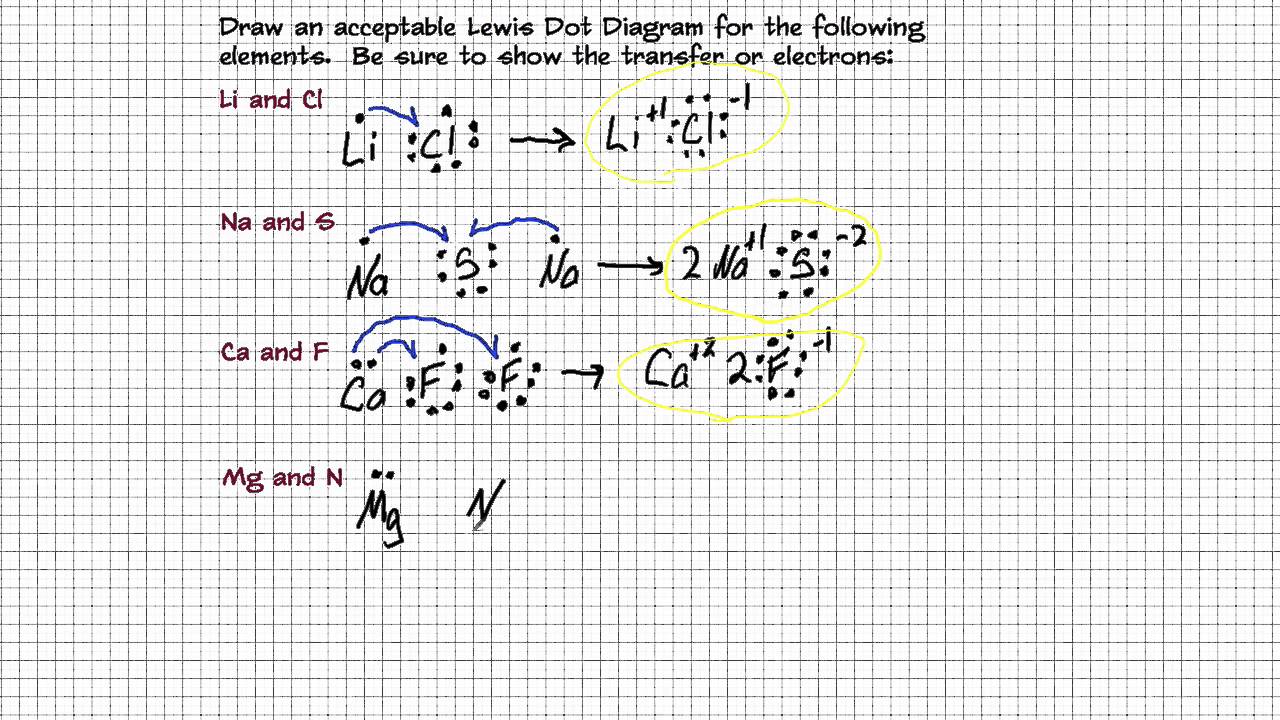
Drawing Lewis Dot Diagrams for Ionic Compounds YouTube

How to Draw a Lewis Structure
![[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams](https://image.slideserve.com/334634/lewis-dot-structure-of-clo-4-by-bonds-table-l.jpg)
[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams
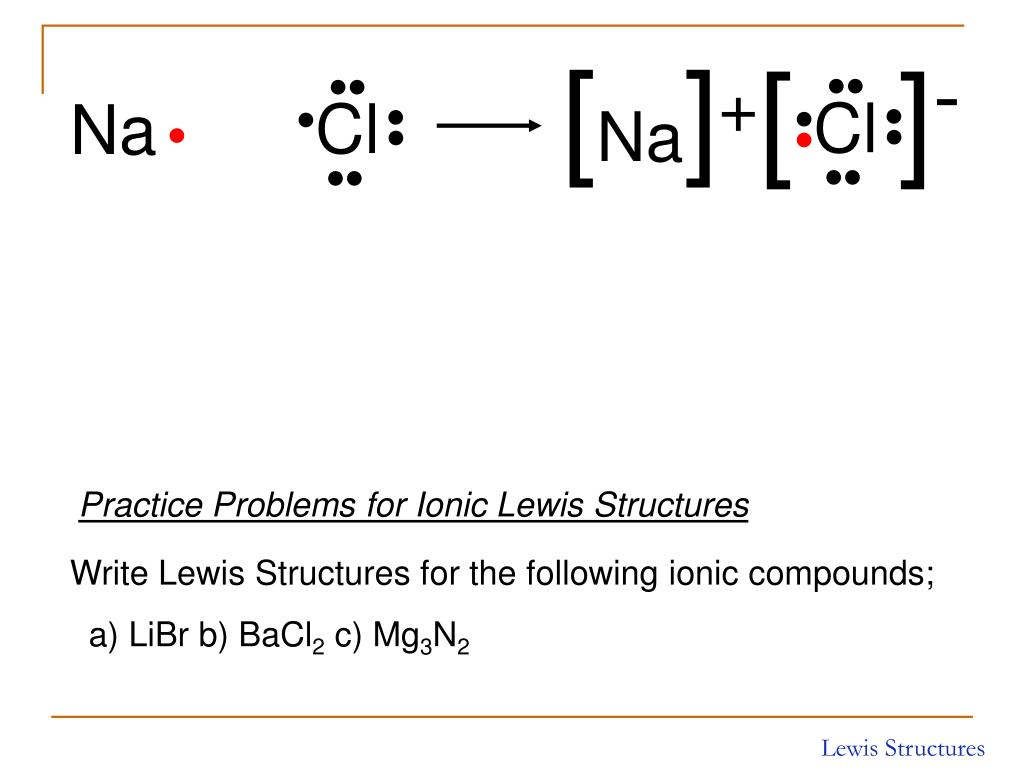
Lewis Structure Of Ionic Compounds

Lewis Diagrams Made Easy How to Draw Lewis Dot Structures Watch
![[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams](https://i.ytimg.com/vi/8wNcKbah62Q/maxresdefault.jpg)
[DIAGRAM] Ionic Bond Drawing Lewis Dot Diagrams
How To Draw Lewis Dot Diagrams Simplereality27

savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures

Formation of Ionic Compounds using Dot Structures YouTube
8.2 How To Draw Lewis Dot Structures | Complete Guide | General Chemistry.
The Diagram Is Also Called A Lewis Dot Diagram, Lewis Dot Formula, Or Electron Dot Diagram.
Web How Can I Draw Lewis Dot Structures For Ionic Compounds?
Web By Drawing A Lewis Dot Structure, You Can Find The Lone Electron Pairs In Molecules, Which Helps You Figure Out How Chemical Bonds Form.
Related Post: