How To Draw Lewis Dot Structure
How To Draw Lewis Dot Structure - A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web how to create a lewis dot structure. Web here are the steps to draw a lewis structure. 8.2 how to draw lewis dot structures | complete guide | general chemistry. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Chad explains and demonstrates exactly how to draw. The first thing we would need to do is to find the total number of valence electrons. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose. Give examples for molecules and ions that do not follow the octet rule. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Chad explains and demonstrates exactly how to draw. Draw resonance structures of some molecules. Using lewis structures to show valence electrons. The diagram is also called a lewis dot diagram, lewis dot formula, or electron. The video covers the basic lewis structures you'll see in an introductory chemistry class. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. 8.2 how to draw lewis dot structures | complete guide | general chemistry. When constructing a lewis diagram, keep in mind. Give examples for molecules and ions that do not follow the octet rule. Web here's some of the guidelines for drawing dot structures. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Also, helium is shown in group 8a, but it only has two valence electrons. Assign. Find the total number of valence electrons. Assign formal charge to an atom in a dot structure. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web here are the steps to draw a lewis structure. Using the periodic table to draw lewis dot. Web here's some of the guidelines for drawing dot structures. Find the total number of valence electrons. Using the periodic table to draw lewis dot structures. The video covers the basic lewis structures you'll see in an introductory chemistry class. Give examples for molecules and ions that do not follow the octet rule. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose. To draw the lewis structure, you will need to know the total number of valence electrons present. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. The video covers the basic lewis. The video covers the basic lewis structures you'll see in an introductory chemistry class. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Learn from anywhereunique learning journeylearn in minutesprompts & resources Web how to create a lewis dot structure. Web a video tutorial for how to draw lewis structures in five steps. The first thing we would need to do. Using lewis structures to show valence electrons. The first thing we would need to do is to find the total number of valence electrons. Web here's some of the guidelines for drawing dot structures. 5 star ratedpaperless solutionstrusted by millionspaperless workflow So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Learn from anywhereunique learning journeylearn in minutesprompts & resources Assess the stability of a structure by considering formal charges of atoms. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web here are the steps to draw a lewis structure. The video covers the basic lewis structures you'll see in an introductory chemistry class. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose. Learn from anywhereunique learning journeylearn in minutesprompts & resources The number of valence electrons in an individual atom can be found based on the atom’s group number in the periodic table. The example is for the nitrate ion. Give examples for molecules and ions that do not follow the octet rule. Web here's some of the guidelines for drawing dot structures. The first thing we would need to do is to find the total number of valence electrons. Web how to create a lewis dot structure. Chad explains and demonstrates exactly how to draw. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Also, helium is shown in group 8a, but it only has two valence electrons. Assign formal charge to an atom in a dot structure.
How to draw Lewis Dot Structure Online Chemistry Tutor

3 Ways to Draw Lewis Dot Structures wikiHow

How to draw Lewis Structures a step by step tutorial Middle School
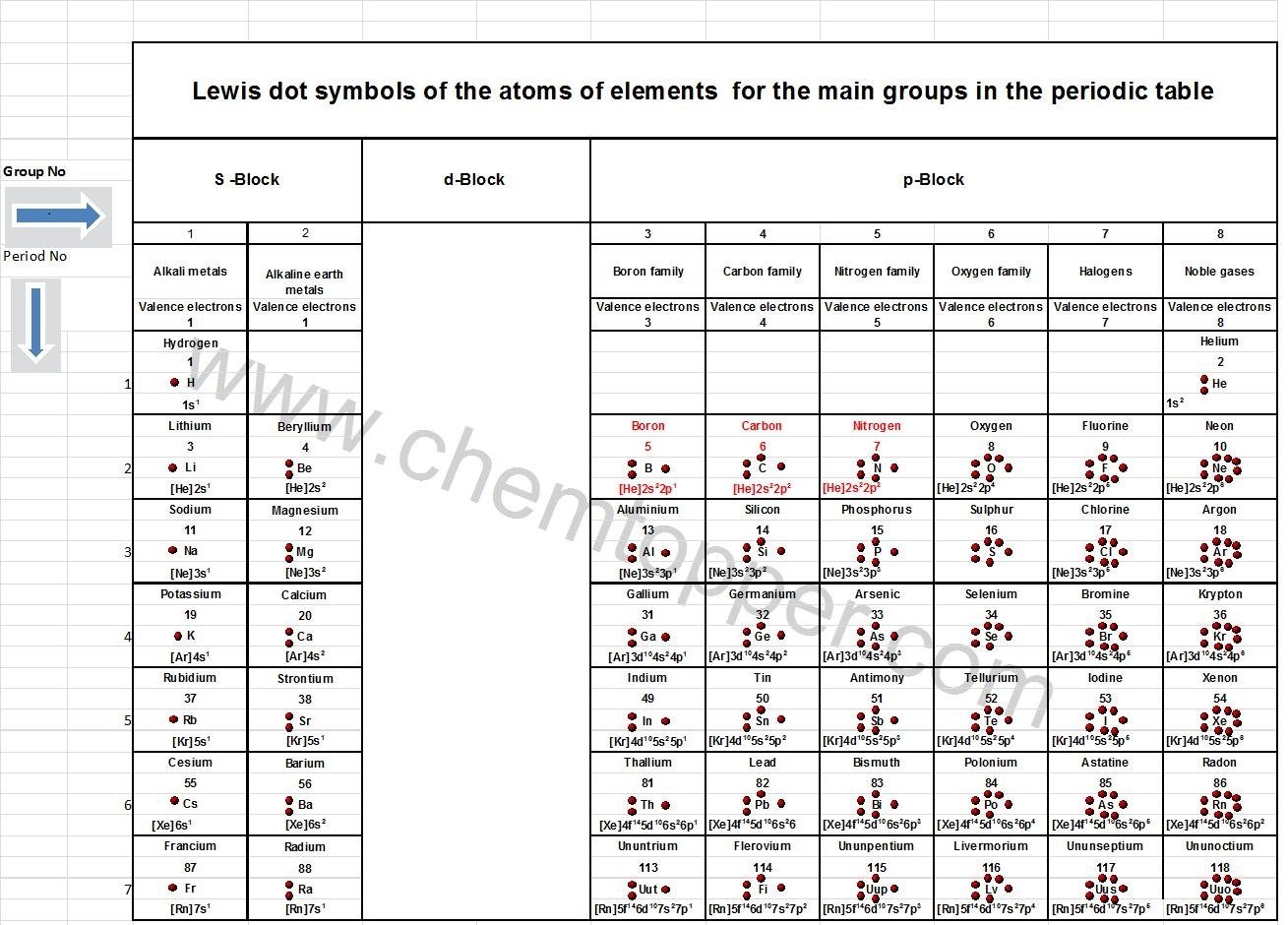
How to Draw Lewis Dot Structure Online Chemistry Tutor

How to Draw a Lewis Structure

Lewis Dot Structure Definition, Examples, and Drawing
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure

Drawing Lewis Dot Structures for Chemistry dummies

3 Ways to Draw Lewis Dot Structures wikiHow

How to Draw a Lewis Structure
8.2 How To Draw Lewis Dot Structures | Complete Guide | General Chemistry.
Assess The Stability Of A Structure By Considering Formal Charges Of Atoms.
To Draw The Lewis Structure, You Will Need To Know The Total Number Of Valence Electrons Present.
Using The Periodic Table To Draw Lewis Dot Structures.
Related Post: