How To Draw Electron Orbitals
How To Draw Electron Orbitals - In this case, sulfur has 16 electrons that need to be placed into orbitals. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Each orbital can hold two electrons. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. Web we're going to look at what orbitals are, what they represent, how electrons go in orbitals, the order electrons go in orbitals, and the shapes of orbitals. Electron configurations are expressed through a notation that looks like this: 2) looking at our cheat sheet, draw the orbitals one at a time, adding electrons as you go, until you reach a total of 16 electrons. If there are more electrons after the 1s, and 2s orbitals have been filled, each p orbital will be filled with one electron first before two electrons try to reside in the same p orbital. Web at ordinary temperatures, the electron in a hydrogen atom is almost invariably found to have the lowest energy available to it. Electron configuration of. Web orbitals can be represented as boxes with the electrons in them shown as arrows. That is, the electron occupies the 1s orbital. Web electron orbitals are mathematical functions that describe the probability of finding an electron around the nucleus of an atom. The energy of atomic orbitals increases as the principal quantum number, n n, increases. In this case,. Orbital energies and atomic structure. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. Web electron configurations and the periodic table. Web for example, the 2p shell has three p orbitals. The periodic table is a helpful tool in writing these configurations. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. The aufbau principle, the pau. Orbital diagrams must follow 3 rules: Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various. Electron configurations describe where electrons are located around the nucleus of an atom. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. You will also learn how to use hund'. Web electron configurations and the periodic table. Remember, arrows represent electrons, and lines or boxes are the orbitals. Web the electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Orbital diagrams must follow 3 rules: Web electron configurations and the periodic table. The aufbau principle, the pau. It looks something like this. The two colors show the phase or sign of the wave function in each region. Electron configuration of nitrogen and oxygen atoms Web electron configurations and the periodic table. Although such drawings show the relative sizes of the orbitals, they do not normally show the spherical nodes in the 2 s and 3 s orbitals because the. Electron configurations and. Web for example, the 2p shell has three p orbitals. Remember, arrows represent electrons, and lines or boxes are the orbitals. Web at ordinary temperatures, the electron in a hydrogen atom is almost invariably found to have the lowest energy available to it. Although such drawings show the relative sizes of the orbitals, they do not normally show the spherical. Each picture is domain coloring of a ψ (x, y, z) function which depends on the coordinates of one electron. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. 1s 2 2s 2 2p 1. Orbital diagrams are a visual way to show where the electrons are located within an. If there are more electrons after the 1s, and 2s orbitals have been filled, each p orbital will be filled with one electron first before two electrons try to reside in the same p orbital. Orbital diagrams are a visual way to show where the electrons are located within an atom. Web we're going to look at what orbitals are,. Web the filling order follows: Remember, arrows represent electrons, and lines or boxes are the orbitals. Web electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. They are also known as atomic orbitals. The periodic table is a helpful tool in writing these configurations. A 1s orbital holding 2 electrons would be drawn as shown on the right, but it can be written even more quickly as 1s 2. Orbital diagrams must follow 3 rules: This is sometimes called the bohr, or the ‘solar system’, model. The two colors show the phase or sign of the wave function in each region. This is known as hund's rule. Web this video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. The energy of atomic orbitals increases as the principal quantum number, n n, increases. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. Web 1) look at the periodic table to see how many electrons sulfur has. Electron configuration of nitrogen and oxygen atoms The shapes of the first five atomic orbitals are: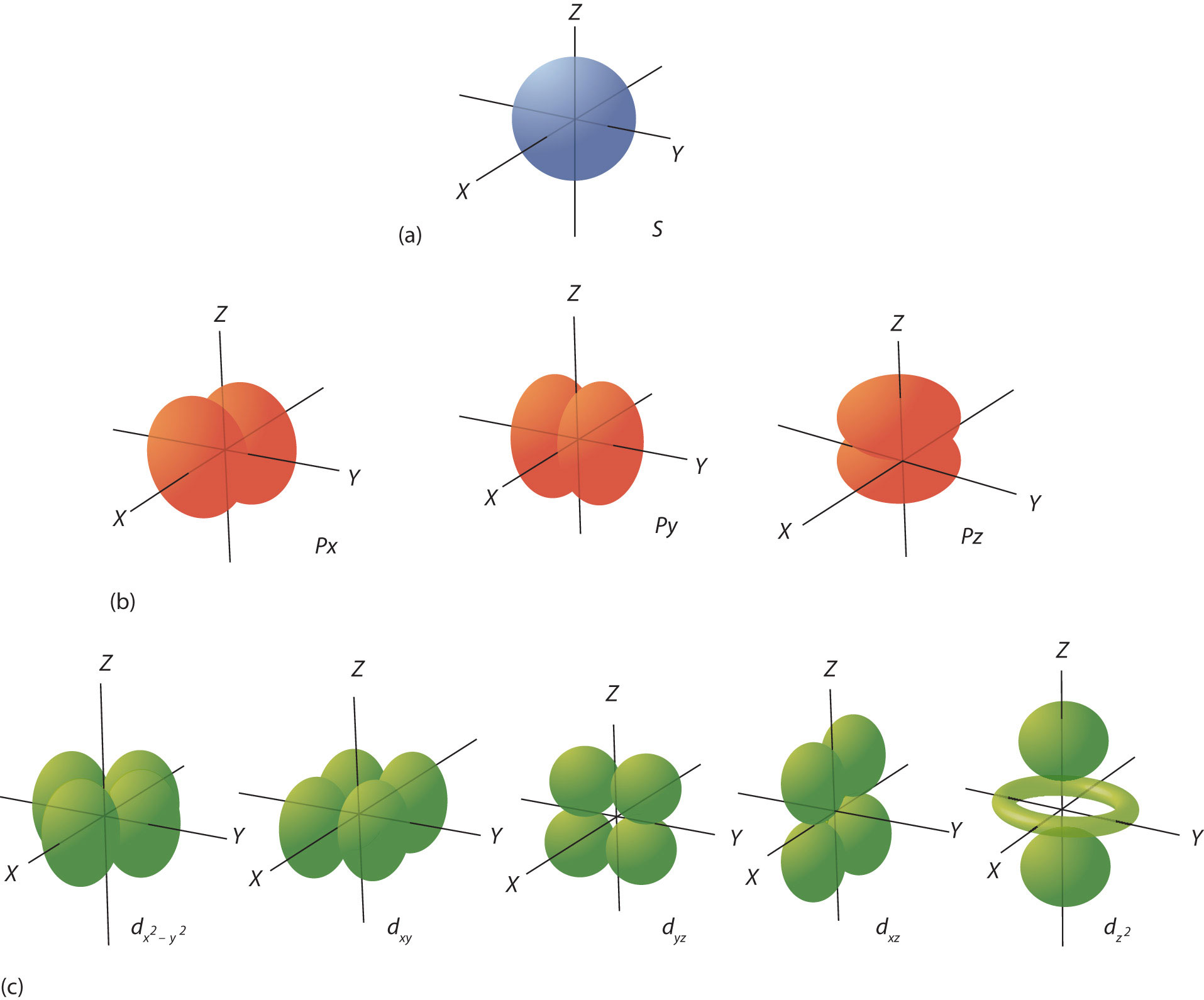
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts

8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
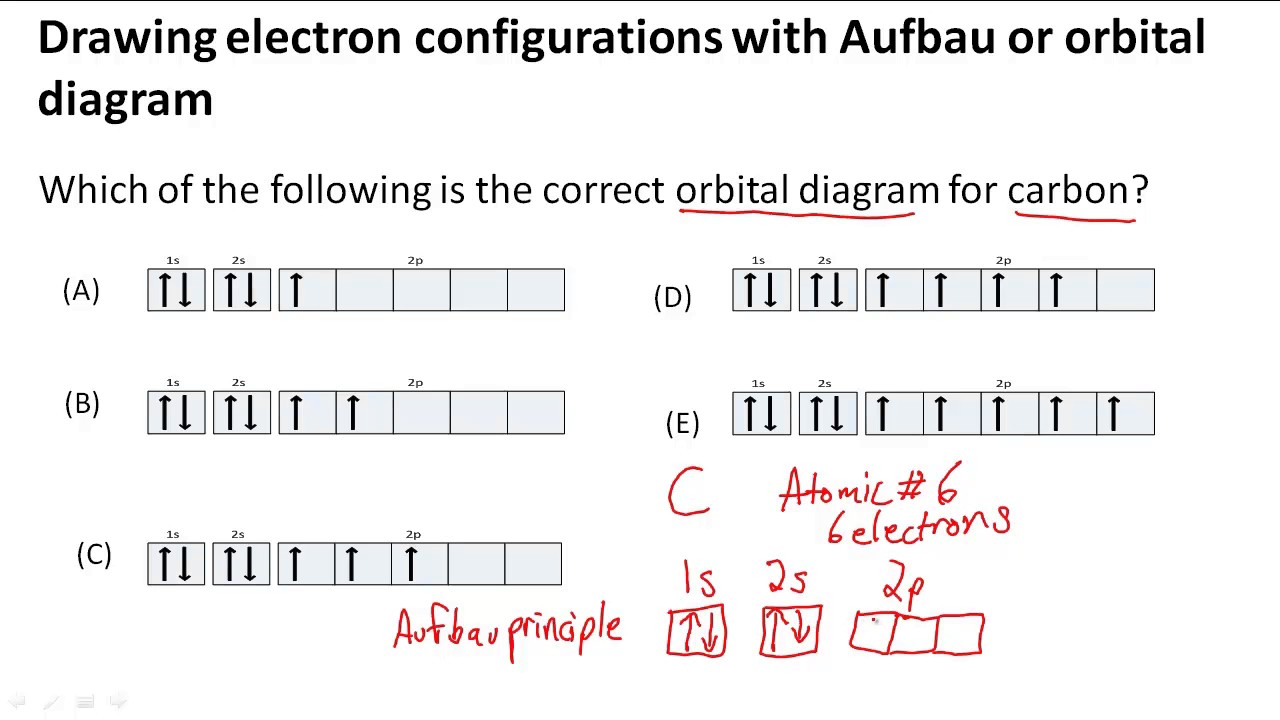
Drawing electron configurations with Aufbau/orbital diagram YouTube

Biochemistry Glossary Orbitals 2. Shape Draw It to Know It
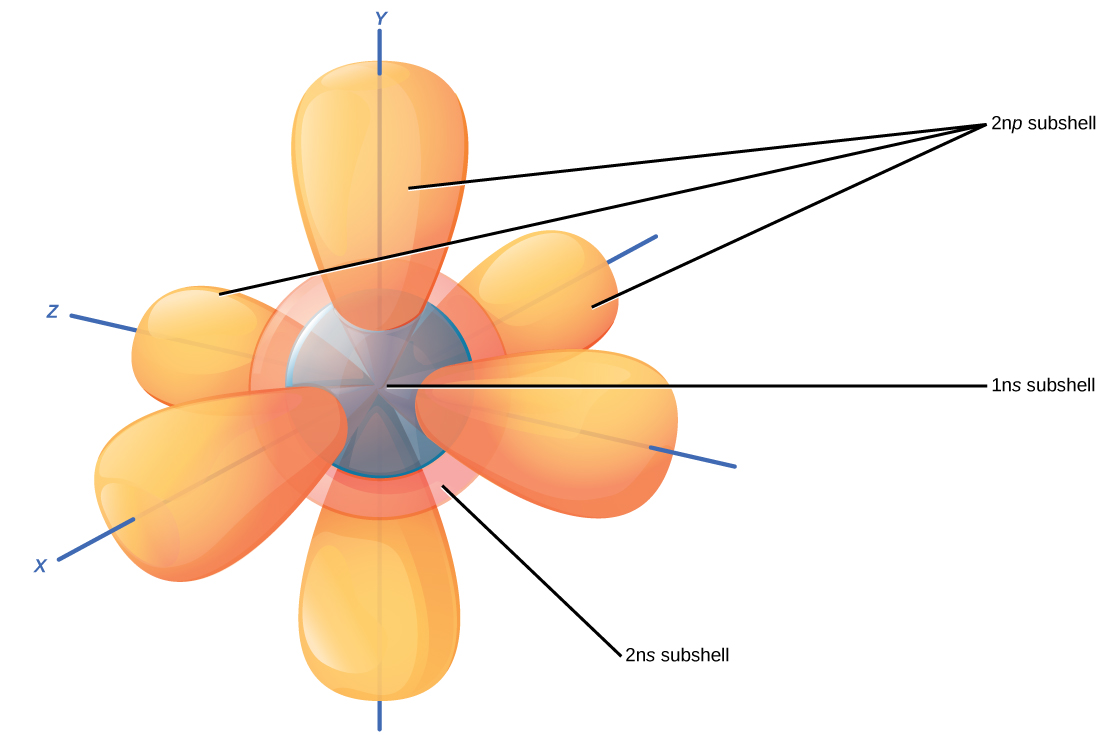
Electrons Biology for Majors I
![Distribution of Electrons in Different Orbits [with Examples] Teacho](https://d77da31580fbc8944c00-52b01ccbcfe56047120eec75d9cb2cbd.ssl.cf6.rackcdn.com/00d8e8eb-2904-4147-abf9-6d87a6c24f05/14.-orbits-teachoo-01.png)
Distribution of Electrons in Different Orbits [with Examples] Teacho

Shapes of Atomic Orbitals — Overview & Examples Expii
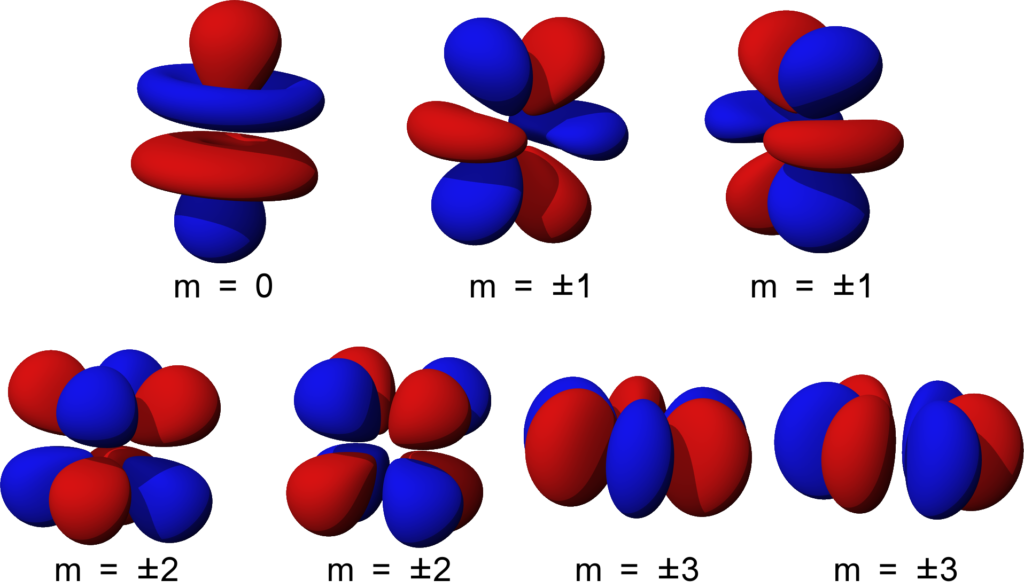
Shapes of Orbitals and their Types Chemistry Skills
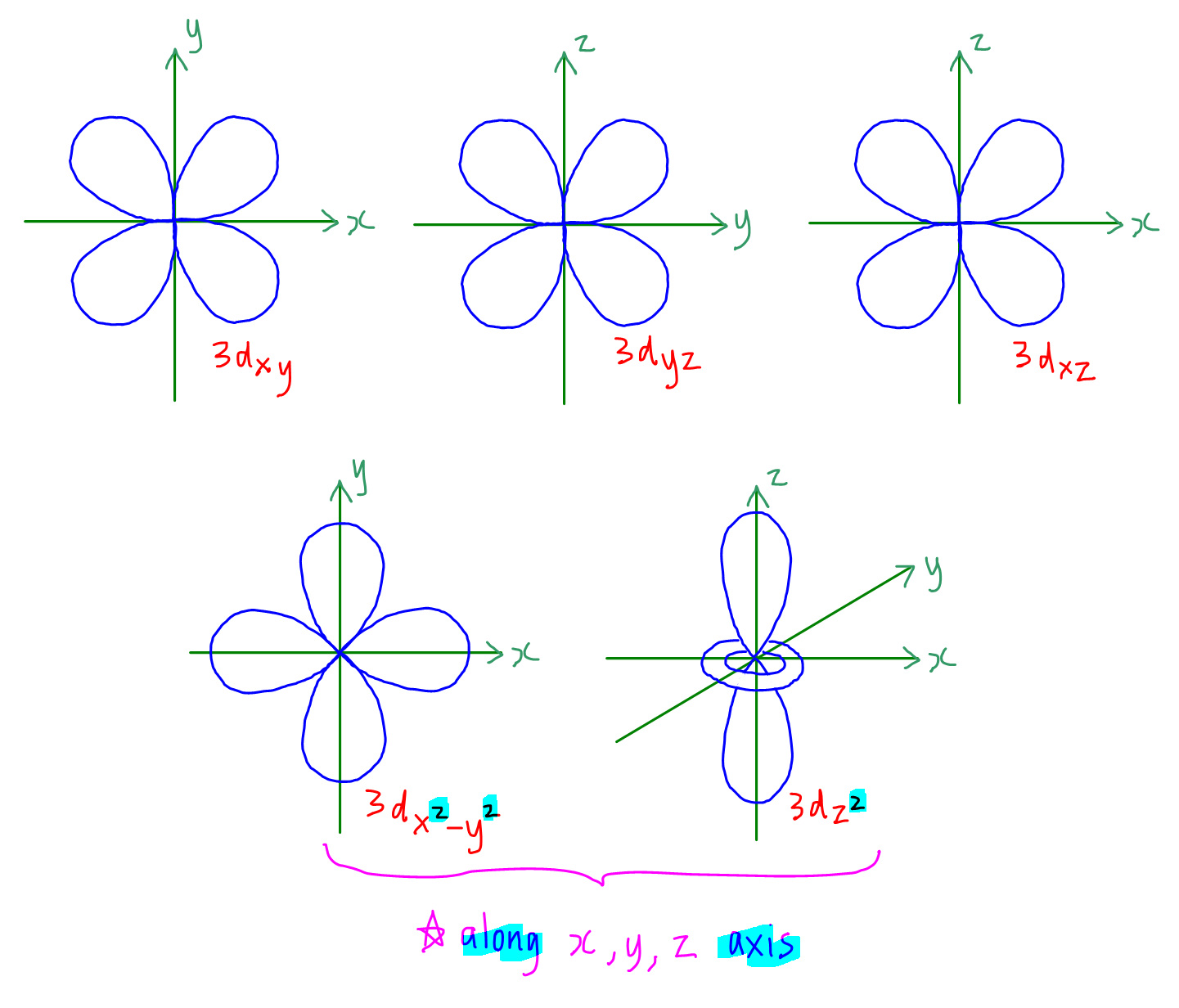
How to Draw Shapes of Orbitals
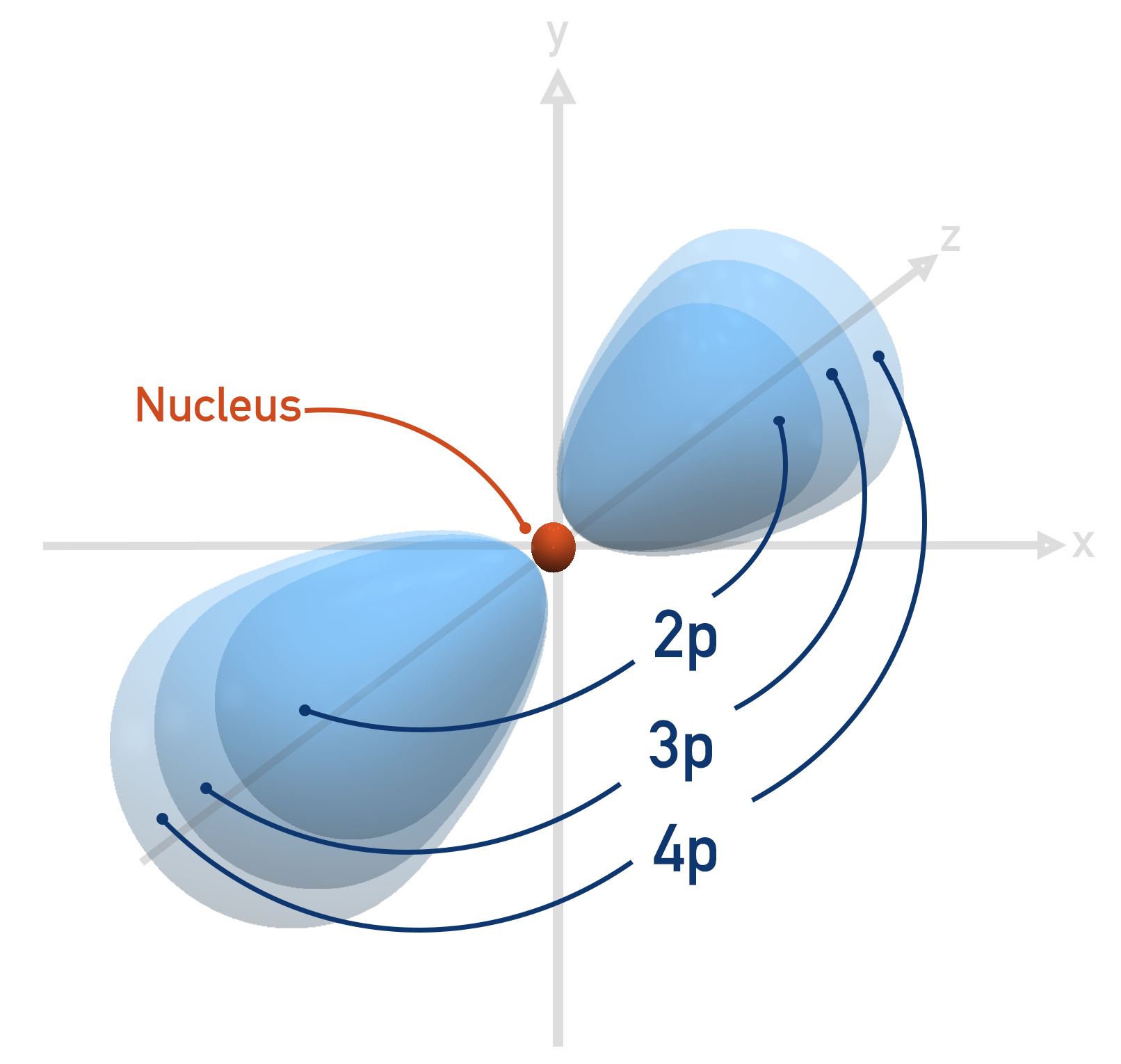
Electron Orbitals (ALevel) ChemistryStudent
Web The Electrons In An Atom Are Arranged In Shells That Surround The Nucleus, With Each Successive Shell Being Farther From The Nucleus.
Web Electron Orbitals Are Mathematical Functions That Describe The Probability Of Finding An Electron Around The Nucleus Of An Atom.
Web For Example, The 2P Shell Has Three P Orbitals.
1S 2 2S 2 2P 1.
Related Post: