How To Draw A Conjugate Base
How To Draw A Conjugate Base - Substances that can serve as both acids and bases, such as water, are. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. The strength of the base depends on its rate of. In the case of [co(nh 3) 5oh 2]3 + reacting under basic conditions, the hydroxide removes a proton from one. Let us take the example of bicarbonate ions reacting with water to create. The structural formulas of five compounds, a through e, are drawn below. A conjugate acid is formed when a proton is added to a base, and a. Draw the conjugate bases for each acid. The stronger an acid, the weaker its conjugate. Web under basic conditions the mecahnism is catalyzed by hydroxide ion. That gives this oxygen a. Web under basic conditions the mecahnism is catalyzed by hydroxide ion. In the case of [co(nh 3) 5oh 2]3 + reacting under basic conditions, the hydroxide removes a proton from one. Web draw the conjugate bases and several resonance structures of each. 856k views 7 years ago new ap & general chemistry video playlist. 856k views 7 years ago new ap & general chemistry video playlist. The structural formulas of five compounds, a through e, are drawn below. Web hcl + h 2 o ↔ cl − + h 3 o +. Web draw the structure of ascorbate, the conjugate base of ascorbic acid, then draw a second resonance contributor showing how the negative. Draw the conjugate bases for each acid. The stronger an acid, the weaker its conjugate. A conjugate base is basically an acid that lost its hydrogen ion. The structural formulas of five compounds, a through e, are drawn below. Web so let's draw in the conjugate base. A conjugate base is basically an acid that lost its hydrogen ion. Here, the chloride anion, cl −, is the conjugate base. This oxygen would have three lone pairs of electrons, and one of those lone pairs would be the electrons in magenta. The strength of the base depends on its rate of. Pka trends for allylic and propargylic (a. Substances that can serve as both acids and bases, such as water, are. In the case of [co(nh 3) 5oh 2]3 + reacting under basic conditions, the hydroxide removes a proton from one. Web \(\mathrm{{\color{red} acid} = h^+ + {\color{blue} conjugate\: Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new. Here, the chloride anion, cl −, is the conjugate base. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Web draw the structure of ascorbate, the conjugate base of ascorbic acid, then draw a second resonance contributor showing how the negative charge is delocalized. Here, the chloride anion, cl −, is the conjugate base. Let us take the example of bicarbonate ions reacting with water to create. Web under basic conditions the mecahnism is catalyzed by hydroxide ion. That gives this oxygen a. What is a conjugate base example? The strength of the base depends on its rate of. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. \(\mathrm{{\color{blue} nh_3} + h_2o \rightleftharpoons {\color{red} nh_4^+}. Sulfuric acid, h 2 so 4 forms two conjugate bases as hydrogen ions are successively. Draw the conjugate bases for each acid. Pka trends for allylic and propargylic (a to an alkyne) systems: Its formula is the acid. Web so let's draw in the conjugate base. Here, the chloride anion, cl −, is the conjugate base. You may select any one of these by clicking the radio button beneath the. Pka trends for allylic and propargylic (a to an alkyne) systems: Substances that can serve as both acids and bases, such as water, are. 856k views 7 years ago new ap & general chemistry video playlist. Web draw the conjugate bases and several resonance structures of each. Sulfuric acid, h 2 so 4 forms two conjugate bases as hydrogen ions. Pka trends for allylic and propargylic (a to an alkyne) systems: A conjugate base is basically an acid that lost its hydrogen ion. Sulfuric acid, h 2 so 4 forms two conjugate bases as hydrogen ions are successively. Web hcl + h 2 o ↔ cl − + h 3 o +. Draw the conjugate bases for each acid. This oxygen would have three lone pairs of electrons, and one of those lone pairs would be the electrons in magenta. Here, the chloride anion, cl −, is the conjugate base. Let us take the example of bicarbonate ions reacting with water to create. A weak base partially dissociates into hydroxide ions and its conjugate acid at equilibrium. The strength of the base depends on its rate of. Web so let's draw in the conjugate base. The stronger an acid, the weaker its conjugate. What is a conjugate base example? A conjugate acid is formed when a proton is added to a base, and a. The structural formulas of five compounds, a through e, are drawn below. Web under basic conditions the mecahnism is catalyzed by hydroxide ion.
What is the conjugate base of HCN? YouTube

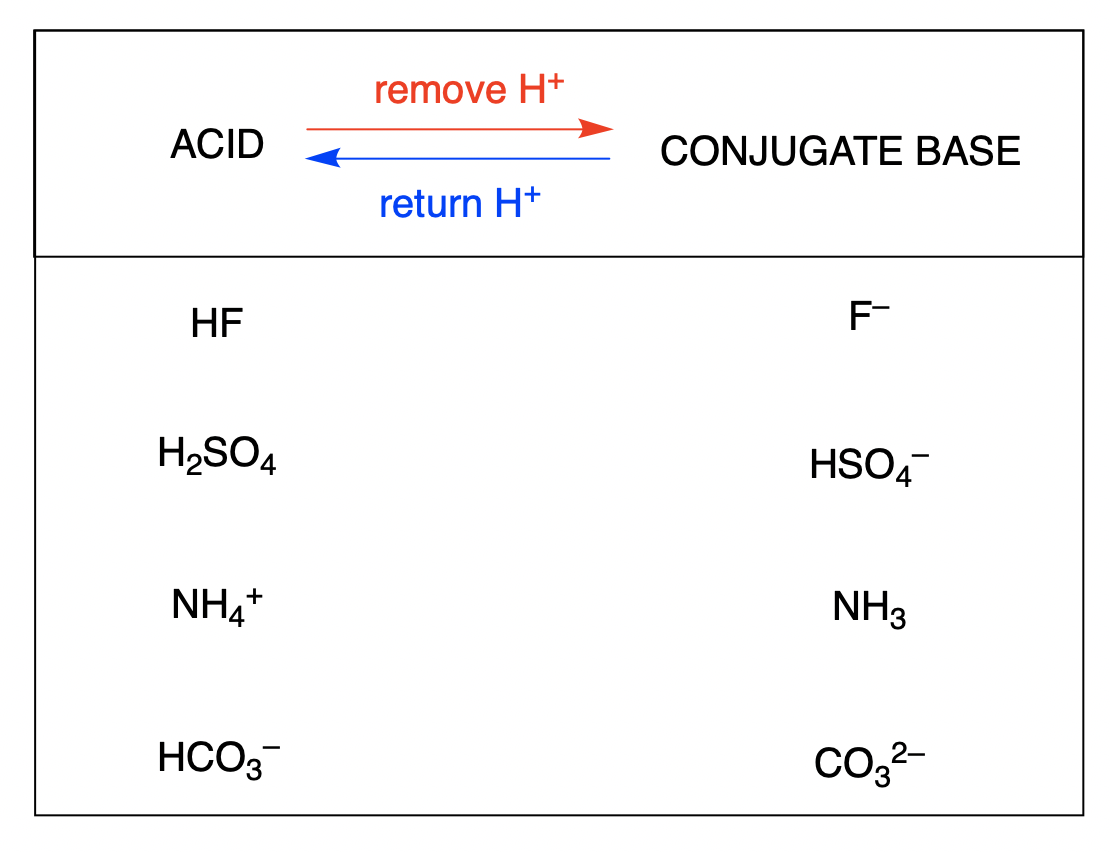
Enter the Conjugate Base for Each Acid.
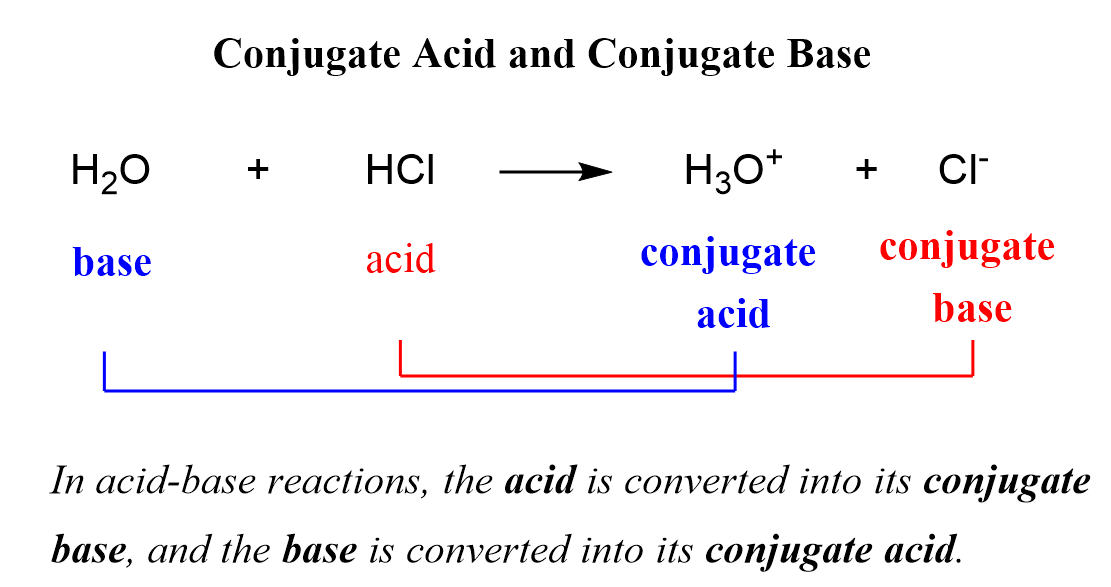
Conjugate Acid and Conjugate Base Chemistry Steps

tikz pgf How to typset/draw conjugate acids and bases TeX LaTeX

Chapter 10 Exercises 3 and 4 Drawing Conjugate Acids and Conjugate
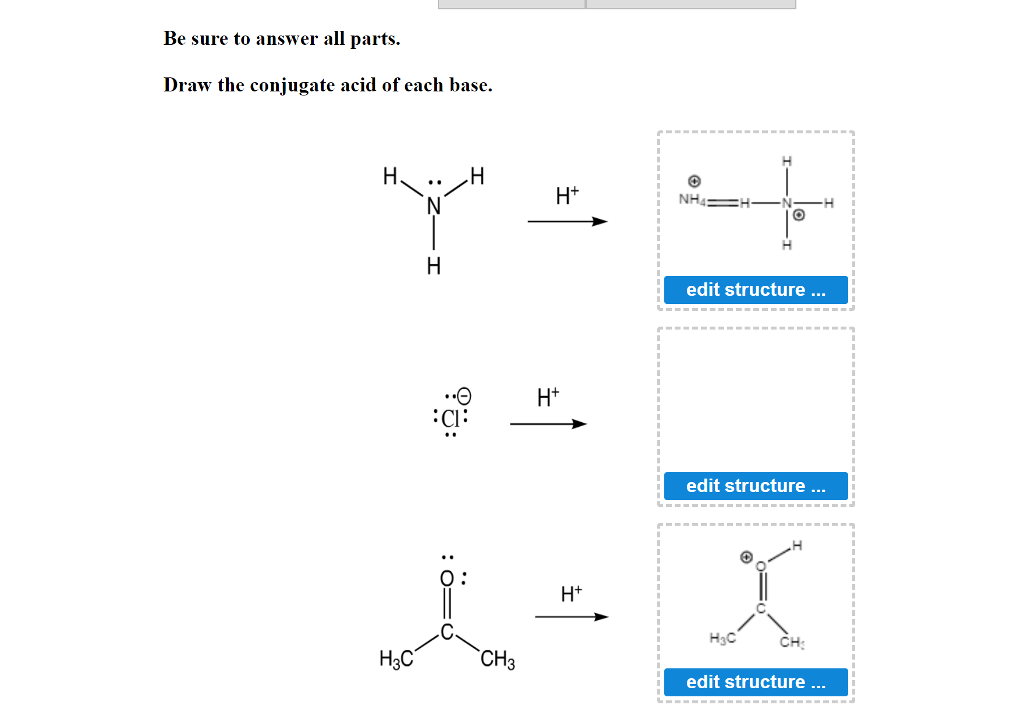
Solved Be sure to answer all parts. Draw the conjugate acid
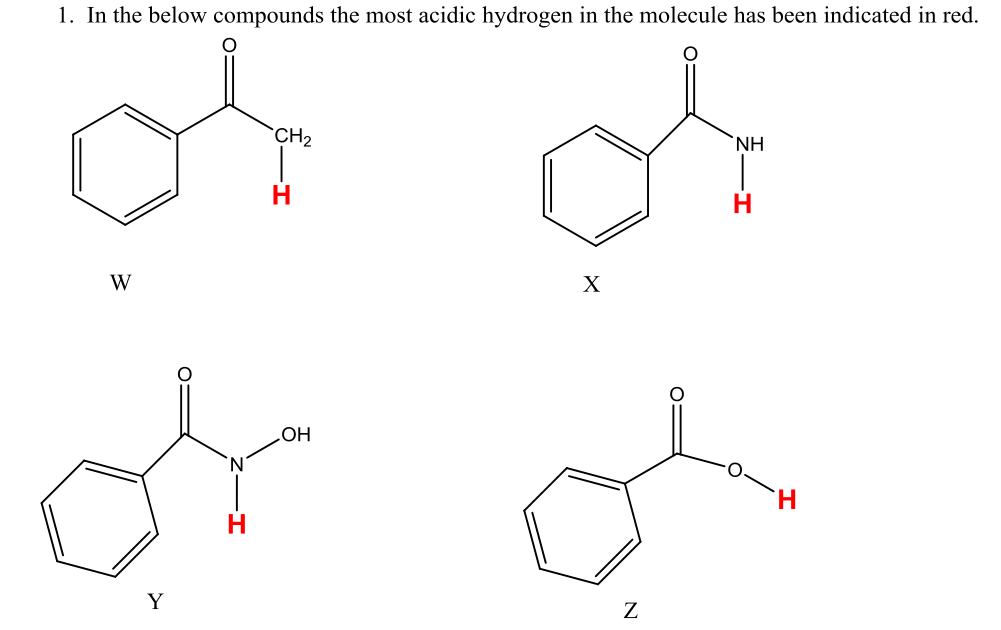
Solved A. Draw the conjugate base of each acid, including
Write the Formula for the Conjugate Acid of Each Base

Trick to Find Conjugate Acid and Conjugate Base in 2 Seconds Super

CHEM112 5 6 drawing conjugate acids and bases YouTube
Substances That Can Serve As Both Acids And Bases, Such As Water, Are.
Web Draw The Conjugate Bases And Several Resonance Structures Of Each.
That Gives This Oxygen A.
Web \(\Mathrm{{\Color{Red} Acid} = H^+ + {\Color{Blue} Conjugate\:
Related Post: