How Do You Draw Resonance Structures
How Do You Draw Resonance Structures - Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Delocalization and resonance structures rules. When there is more than one type of atom, keep the least electronegative or metallic atom as the central atom. Where there can be a double or triple bond, draw a dotted. Notice there is a positive formal charge on the top oxygen and a negative on the oxygen to its left. Calculate the total number of valence electrons from each atom. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis formula. That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. 1) there is only one real structure for each molecule or ion. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. Then, draw in the bond lines and. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). It explains how to identify the major resonance contributor as well as the minor resonance. However, a single lewis structure cannot explain chemical bonding due to partial charges on the atoms and fractional bond order. We just need a graphical tool to do it. We can. Web it is useful to combine the resonance structures into a single structure called the resonance hybrid that describes the bonding of the molecule. However, a single lewis structure cannot explain chemical bonding due to partial charges on the atoms and fractional bond order. When there is more than one type of atom, keep the least electronegative or metallic atom. In following examples, arrows are used to show electrons transformation. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the. In resonance structures, the electrons are able to move to help stabilize the molecule. We can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs. Also, you will learn how to dr. It explains how to identify the major resonance contributor as well as the minor resonance. The general approach is described below: Equivalent lewis structures are called resonance forms. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Web this general chemistry video tutorial provides a basic introduction into resonance structures. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: It explains how to draw the resonance structures using curved. However, a single lewis structure cannot explain chemical bonding due to partial charges on the atoms. In resonance structures, the electrons are able to move to help stabilize the molecule. We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. They are used when there is more than one way to place double bonds and lone pairs on atoms. An animation of how one can do. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Also, you will learn how to dr. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Web it is useful to combine the resonance structures into a single structure called the resonance hybrid that describes the bonding of. All right, let's look at an application of the acetate anion here, and the resonance structures that we can draw. So let's say we wanted to draw a resonance structure for this carbocation. We just need a graphical tool to do it. Web drawing lewis structures: Equivalent lewis structures are called resonance forms. Web introducing curved arrows, a tool for showing the movement of electrons between resonance structures. Web this lecture is about resonance structures in chemistry. Notice there is a positive formal charge on the top oxygen and a negative on the oxygen to its left. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Web ©. How stable are the negative charges? So let's say we wanted to draw a resonance structure for this carbocation. We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. It explains how to identify the major resonance contributor as well as the minor resonance. This real structure (the resonance hybrid ) takes its character from the average of all the individual resonance contributors. Also, you will learn how to dr. This movement of the electrons is called delocalization. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Web drawing lewis structures: Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis formula. Web © 2024 google llc. In resonance structures, it does not require to show transformation of electrons by arrows. However, a single lewis structure cannot explain chemical bonding due to partial charges on the atoms and fractional bond order. But, to identify each resonance structures, it is good to show arrows. How stable are the positive charges? In this video, you'll become an expert at identifying and drawing resonance structures for organic molecules.
How To Draw Resonance Structures YouTube
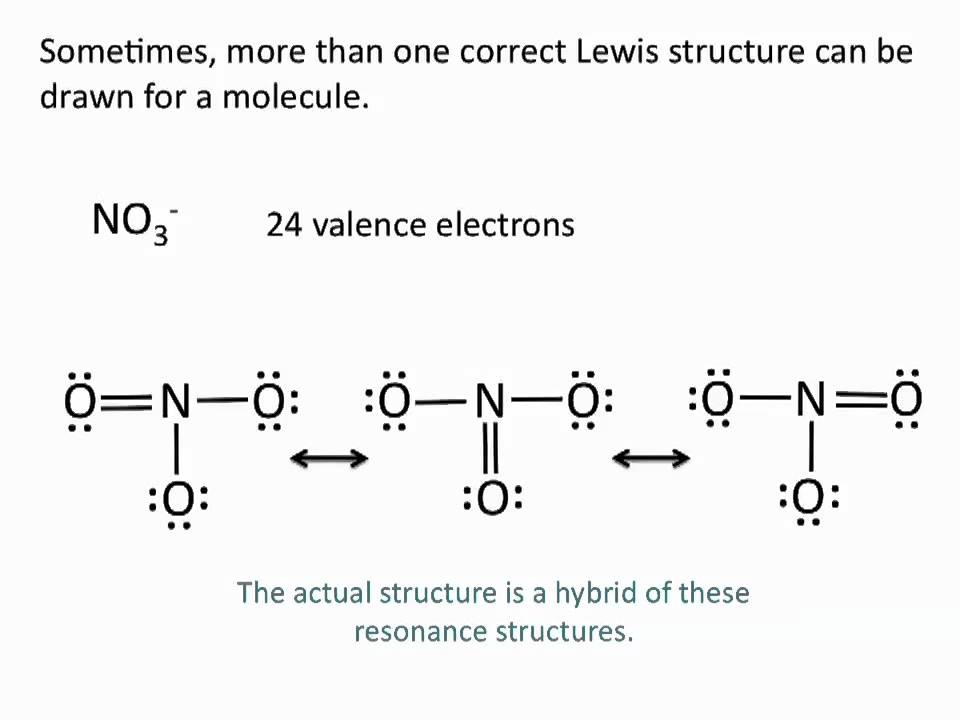
Drawing Lewis Structures Resonance Structures Chemistry Tutorial

3 Ways to Draw Lewis Dot Structures wikiHow

Resonance Structures YouTube
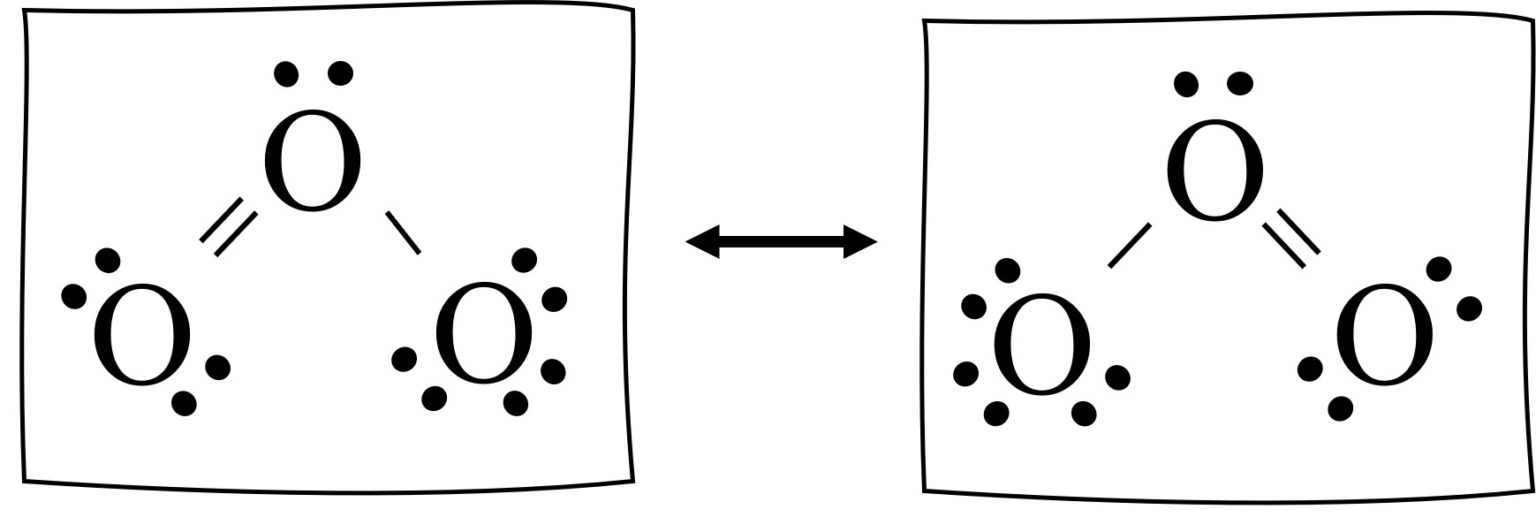
Resonance Structures Easy Hard Science

HOW TO DRAW RESONANCE STRUCTURES YouTube

How to Draw a Lewis Structure

Resonance Structures, Basic Introduction How To Draw The Resonance
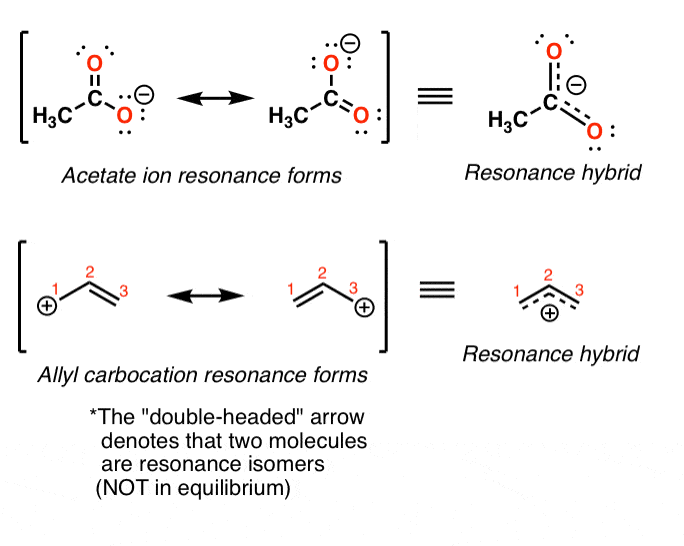
Resonance Structures 4 Rules On How To Evaluate Them, With Practice
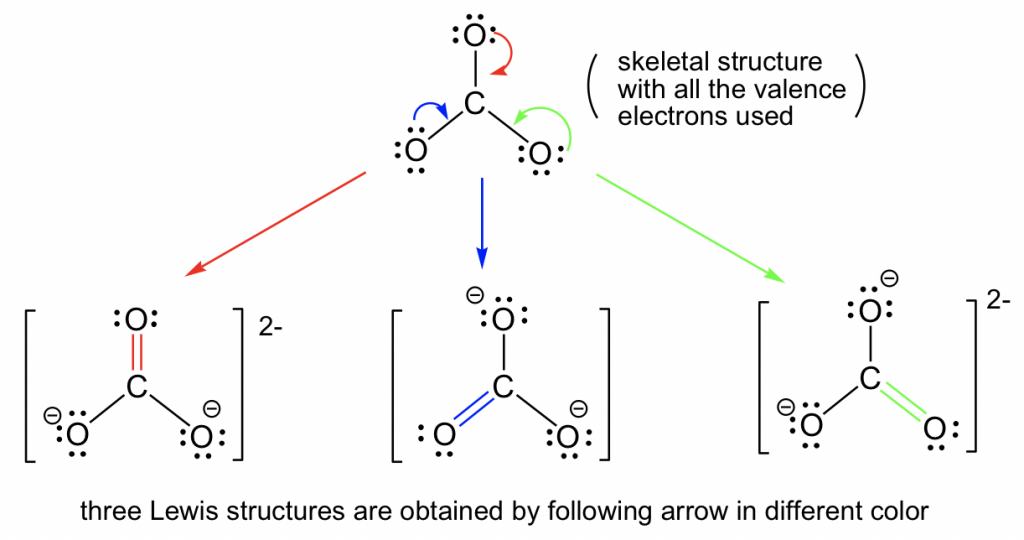
1.3 Resonance Structures Chemistry LibreTexts
This Molecule Has Two Extra Electrons).
Web Introducing Curved Arrows, A Tool For Showing The Movement Of Electrons Between Resonance Structures.
Some Molecules Have Two Or More Chemically Equivalent Lewis Electron Structures, Called Resonance Structures.
An Animation Of How One Can Do A Resonance With Ozone By Moving Electrons.
Related Post: