How Do You Draw A Bohr Rutherford Diagram
How Do You Draw A Bohr Rutherford Diagram - • how to draw any bohr diagram of any element on the periodic table • identify group and period on the periodic table • define: Primmer demonstrates how to draw bohr rutherford diagrams! Since you have 2 electrons already drawn, you need to add 4 more. Bohr's model calculated the following energies for an electron in the shell, n. Find the number of protons, neutrons and electrons for the atom. For example, consider the following diagram for phosphorous: Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The first shell can only hold 2 electrons. You should have 6 total electrons for. Each ring has a maximum number of electrons that it can hold. Web the bohr atomic model, relying on quantum mechanics, built upon the rutherford model to explain the orbits of electrons. Web draw the shells around the nucleus. The atomic mass is the total number of protons plus the total number of neutrons in the nucleus. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence,. You should have 6 total electrons for. This allows you to see how the electrons are arranged around the nucleus and determine the stability of the atom. Bohr diagrams indicate how many electrons fill each principal shell. Draw orbitals around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n. A full valence shell is the most stable electron configuration. There are certain rules to follow when drawing these diagrams: 415k views 12 years ago. Web this lesson will walk your students through the basics on how to draw a bohr diagram for the first 20 elements on the periodic table. • how to draw any bohr diagram of any. Web calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.check me out: Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Primmer demonstrates how to draw bohr rutherford diagrams! Group 18 elements (helium, neon, and argon. • how to draw any bohr diagram of any element on the periodic table • identify group and period on the periodic table • define: Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. In this model, the electrons are. The nucleus is shown as one green circle in the center. Web how did bohr respond to the drawbacks in the rutherford model? Represent electrons as pairs of dots in the orbitals. Primmer demonstrates how to draw bohr rutherford diagrams! This allows you to see how the electrons are arranged around the nucleus and determine the stability of the atom. The atomic mass is the total number of protons plus the total number of neutrons in the nucleus. Web using the bohr rutherford diagram maker, you can input the number of protons, neutrons, and electrons in an atom and generate a diagram that shows the distribution of electrons in different energy levels. Drawing a shell model diagram and an energy. Describe the arrangement of electrons using the shell model. Web draw the shells around the nucleus. Bohr diagrams indicate how many electrons fill each principal shell. Web this lesson will walk your students through the basics on how to draw a bohr diagram for the first 20 elements on the periodic table. A full valence shell is the most stable. Primmer demonstrates how to draw bohr rutherford diagrams! Web using the bohr rutherford diagram maker, you can input the number of protons, neutrons, and electrons in an atom and generate a diagram that shows the distribution of electrons in different energy levels. Bohr’s model of the atom can be combined with rutherford’s model in diagrams that summarize the numbers and. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. Draw electrons as dots on the rings that represent the energy levels. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. 2 electrons can go in the first shell, 8 in. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Calculating electron energy for levels n=1 to 3. The first shell can only hold 2 electrons. Draw orbitals around the nucleus. You should have 6 total electrons for. Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. Primmer demonstrates how to draw bohr rutherford diagrams! • how to draw any bohr diagram of any element on the periodic table • identify group and period on the periodic table • define: A full valence shell is the most stable electron configuration. I also created a simple worksheet for students to record their drawings and do independent practice.… Bohr diagrams indicate how many electrons fill each principal shell. This allows you to see how the electrons are arranged around the nucleus and determine the stability of the atom. 415k views 12 years ago. The nucleus is shown as one green circle in the center. Each ring has a maximum number of electrons that it can hold.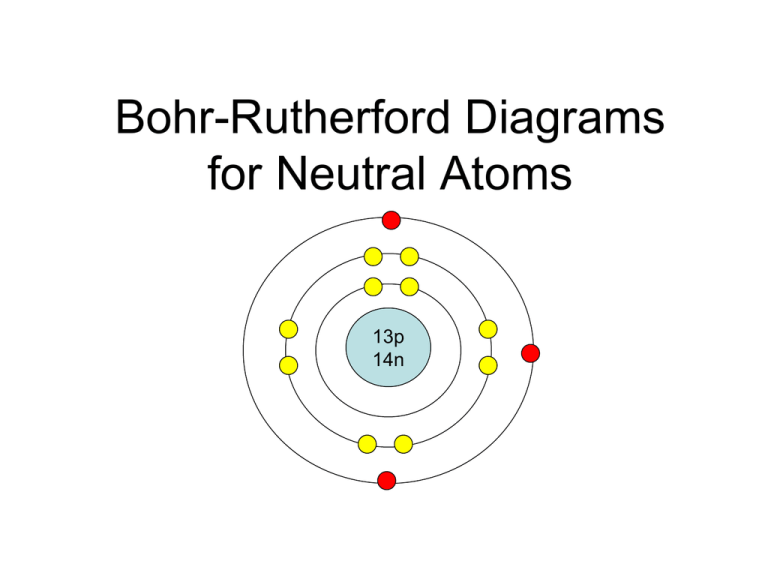
BohrRutherford diagrams for atoms
Bohr Model of the Atom Overview and Examples
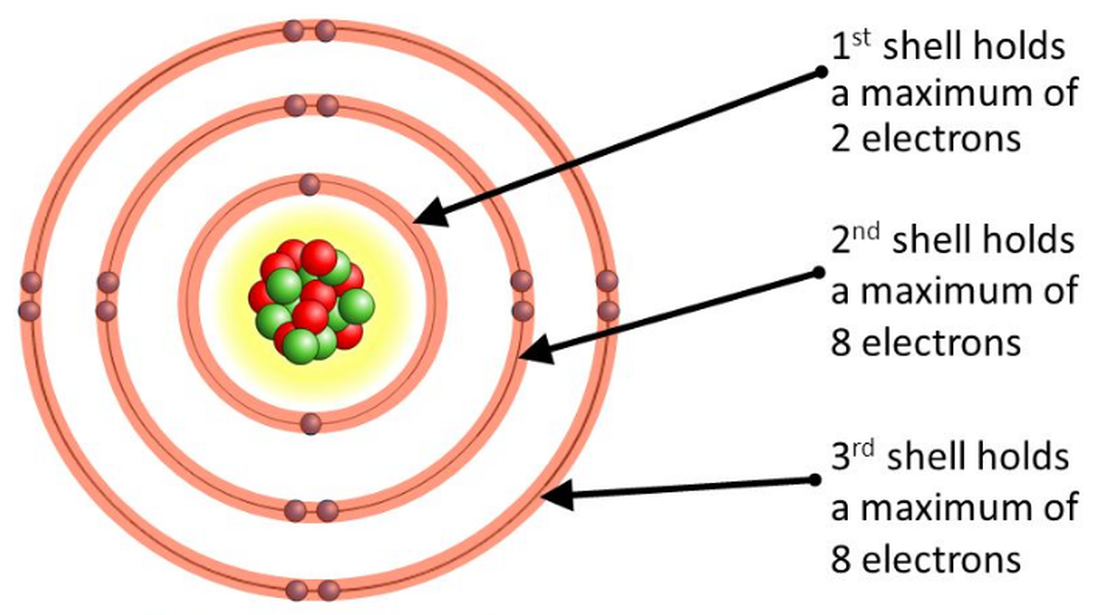
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons

bohr rutherford diagram

Bohr Diagram For Atom

How to Draw a BohrRutherford Diagram YouTube

How to draw a Bohr model of an atom BohrRutherford Diagrams
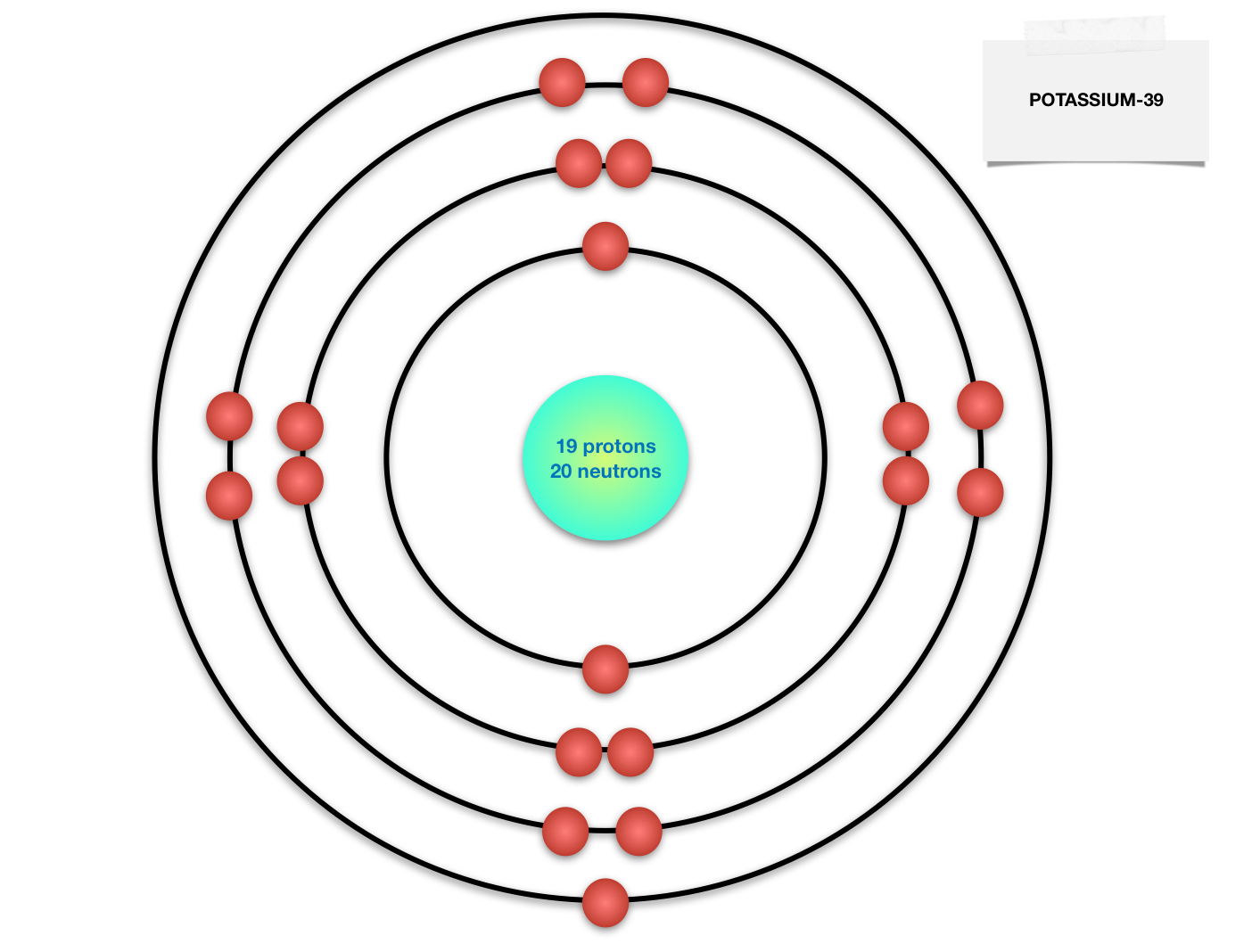
Bohr Diagram For Potassium
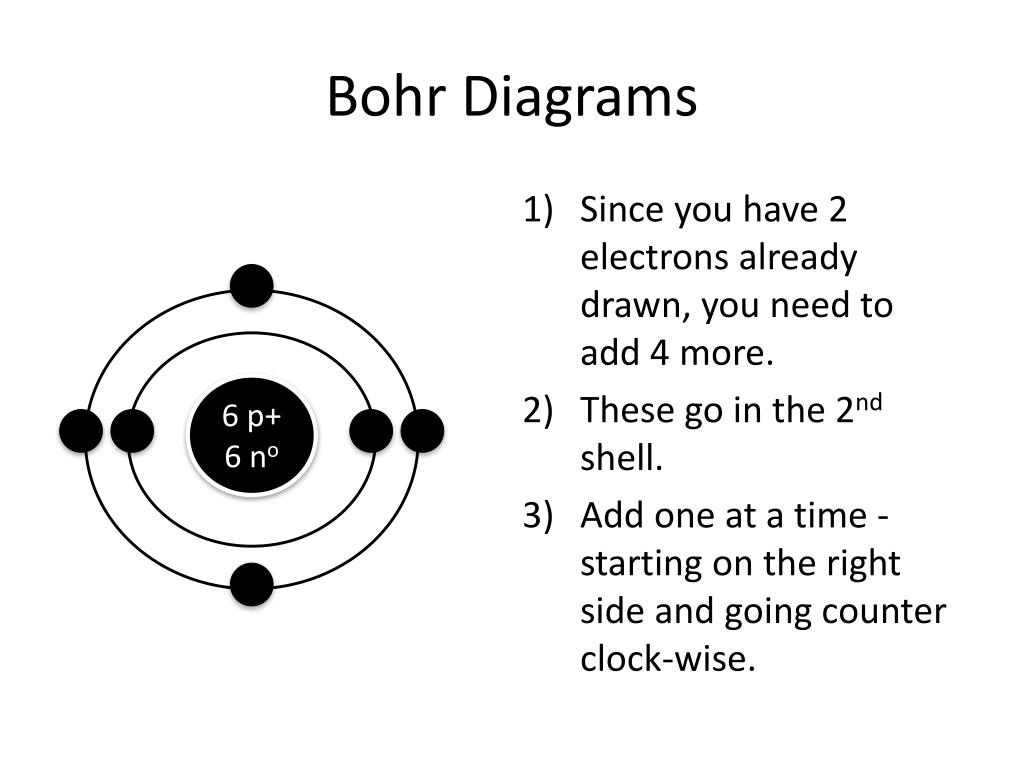
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download

bohr rutherford diagram
Web This Lesson Will Walk Your Students Through The Basics On How To Draw A Bohr Diagram For The First 20 Elements On The Periodic Table.
The Number Of Protons Is The Atomic Number.
Web The Atomic Number Tells (1) The Total Number Of Protons Inside The Nucleus And (2) The Total Number Of Electrons.
Bohr's Model Of Hydrogen Is Based On The Nonclassical Assumption That Electrons Travel In Specific Shells, Or Orbits, Around The Nucleus.
Related Post: