Electronegativity Drawing
Electronegativity Drawing - An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. If you want a wider view of electronegativity. Web bond polarity is due to differences in electronegativity (en), the intrinsic ability of an atom to attract the shared electrons in a covalent bond. It explains how to indicate the polarity of a bond and of a. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. A low electronegativity value means an atom readily donates electrons to form a bond or is electropositive. Web draw the lewis structure; In our previous work we learned why atoms form covalent bonds and how to draw the resulting organization of atoms. The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. Figure out the geometry (using vsepr theory) visualize or draw the geometry; Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Electronegativity differences in bonding using the pauling scale. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. Web the protons in the nucleus of an atom are positively charged, and want to draw in. (b) a is very electronegative, and b and c are not. Web electronegativity determines how the shared electrons are distributed between the two atoms in a polar covalent bond. Web follow this procedure for drawing lewis structures until the process becomes second nature. This handout is also included at the end of this packet. Electronegativity is a function of: Web draw the lewis structure; In this lesson we will learn (a) how the combination of bonded electrons and lone pairs of electrons result in different molecular shapes and (b) how unequal sharing of. Web follow this procedure for drawing lewis structures until the process becomes second nature. This handout is also included at the end of this packet. In. Web electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. Web use the electronegativity controls to determine how the molecular dipole will look for the starting bent molecule if: Web the electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. It. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Web use the electronegativity controls to determine how the molecular dipole will look for the starting bent molecule if: Classifying bonds as covalent, polar covalent, or ionic. Want to join the conversation? A low electronegativity value means an atom readily donates electrons to. In our previous work we learned why atoms form covalent bonds and how to draw the resulting organization of atoms. Classifying bonds as covalent, polar covalent, or ionic. A high electronegativity value means an atom readily attracts electrons to form a chemical bond with another atom. Relatively electronegative atoms, such as fluorine, tend to inductively draw electrons towards themselves and.. (b) a is very electronegative, and b and c are not. Web electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements. What does capitol delta look like. Electronegativity differences in. Electronegativity differences in bonding using the pauling scale. What does capitol delta look like. Electronegativity difference is above 2.0 (metal + nonmetal) Relatively electronegative atoms, such as fluorine, tend to inductively draw electrons towards themselves and. It determines how the shared electrons are distributed between the two atoms in a bond. Electronegativity difference in between 0.4 and 2.0 (nonmetal + nonmental further apart on the periodic table) ionic: Want to join the conversation? Web follow this procedure for drawing lewis structures until the process becomes second nature. This activity supports students’ understanding of. Like bonds, molecules can also be polar. In a polar molecule, electron density is unevenly distributed throughout the molecule, resulting in regions of partial negative charge and regions of partial positive charge. Web bond polarity is due to differences in electronegativity (en), the intrinsic ability of an atom to attract the shared electrons in a covalent bond. (b) a is very electronegative, and b and c are. If you want a wider view of electronegativity. Solution (a) molecular dipole moment points immediately between a and c. Classifying bonds as covalent, polar covalent, or ionic. Change the electronegativity of atoms in a molecule to see how it affects polarity. Web electronegativity is a measure of how easily an atom attracts a pair of electrons to form a chemical bond. Web electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. This page deals with electronegativity in an organic chemistry context. Electronegativity is a function of: The atom's ionization energy (how strongly the atom holds on to its own electrons) and. Relatively electronegative atoms, such as fluorine, tend to inductively draw electrons towards themselves and. Web in this video we look at the concept of electronegativity, how it varies across a period and how to draw lewis dot diagrams for atoms. It determines how the shared electrons are distributed between the two atoms in a bond. (b) a is very electronegative, and b and c are not. What does capitol delta look like. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. Web electronegativity determines how the shared electrons are distributed between the two atoms in a polar covalent bond.Periodic Table of Electronegativities

What is Electronegativity?

Electronegativity Definition and Trend

Electronegativity, Basic Introduction, Periodic Trends Which Element
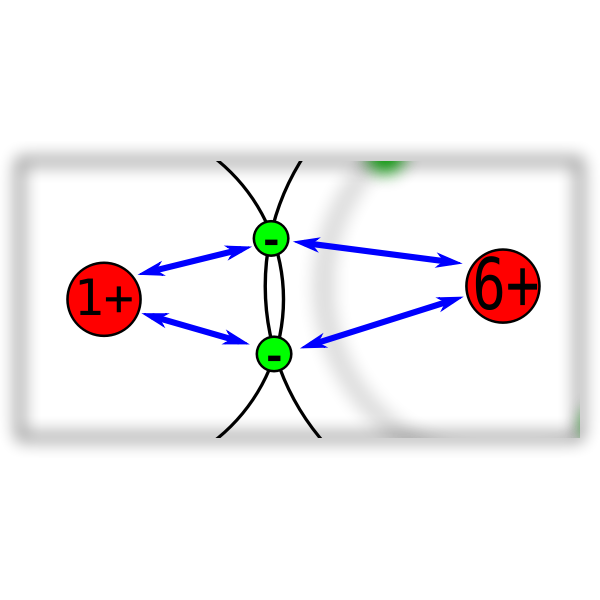
Electronegativity diagram Free SVG
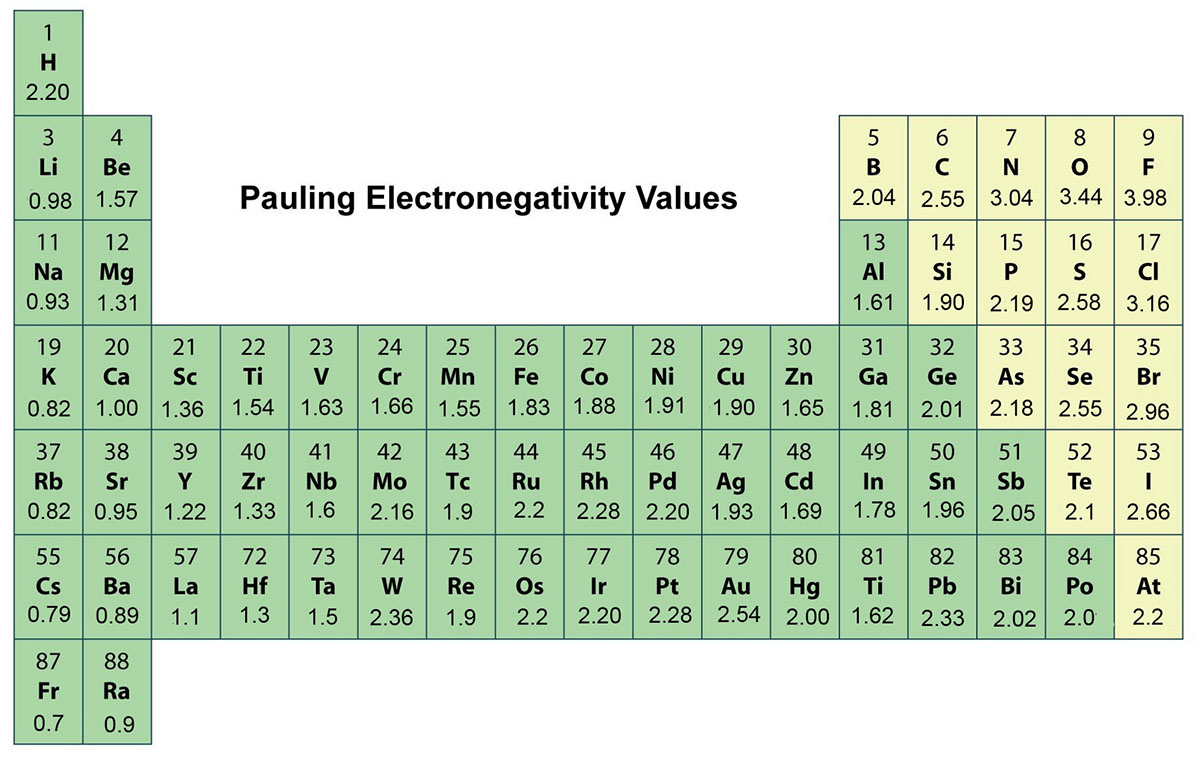
Electronegativity explained
Making Sense of the Electronegativity Chart StudentTutor Education Blog
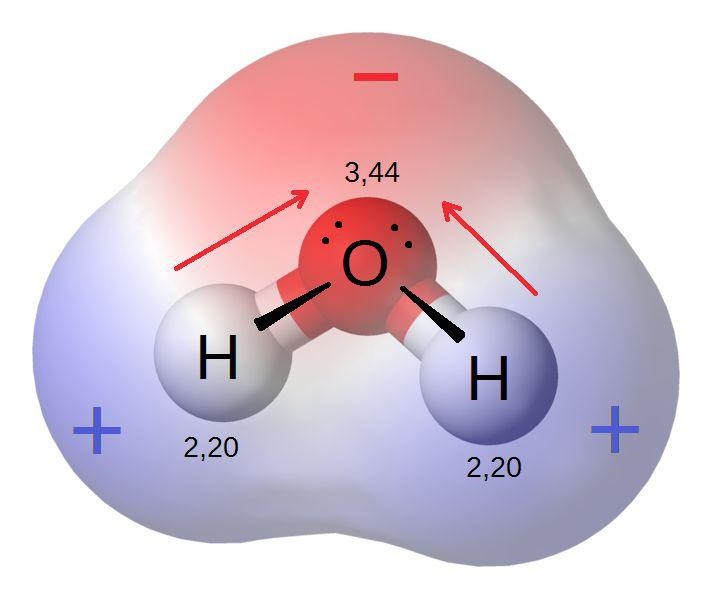
Electronegativity Facts, Summary & Definition Chemistry Revision
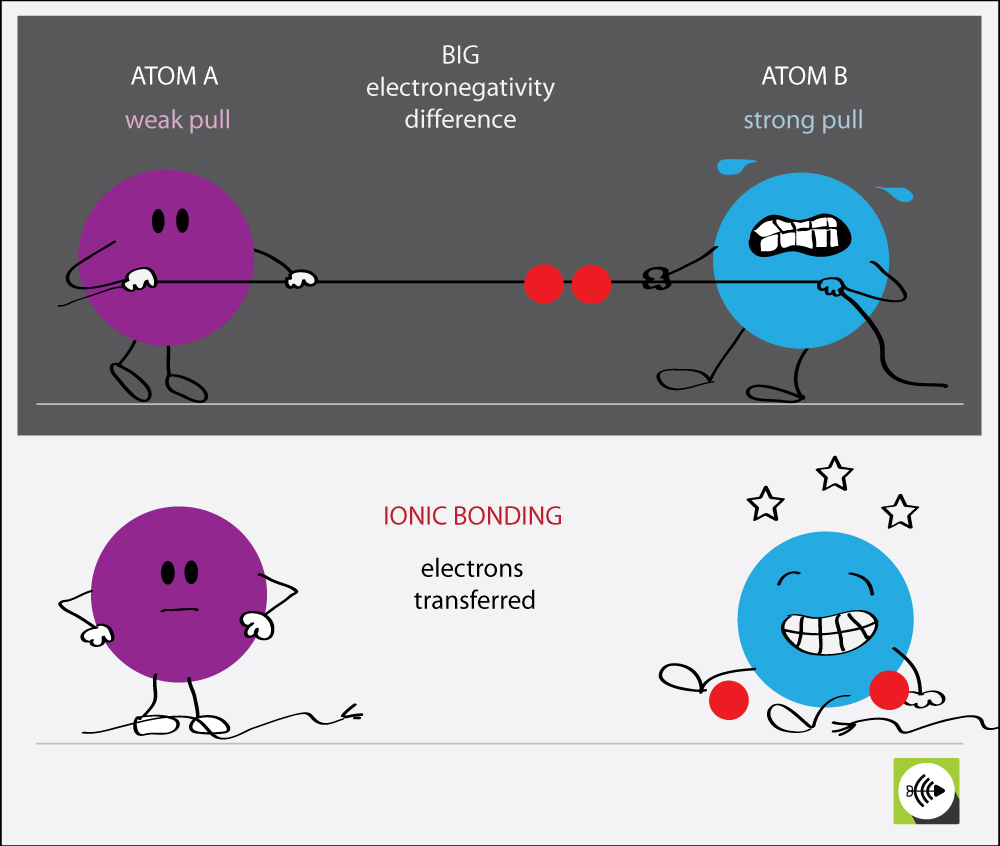
Electronegativity Bond Scale Surfguppy Chemistry made easy for
:max_bytes(150000):strip_icc()/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)
What Is Electronegativity and How Does It Work?
Web Electronegativity Is Defined As The Ability Of An Atom In A Particular Molecule To Attract Electrons To Itself.
As Shown In Figure 2.3 , Electronegativities Are Based On An Arbitrary Scale, With Fluorine The Most Electronegative (En = 4.0) And Cesium The Least (En = 0.7).
Web Draw The Lewis Structure;
In Our Previous Work We Learned Why Atoms Form Covalent Bonds And How To Draw The Resulting Organization Of Atoms.
Related Post:
.PNG)