Drawing Electron Configuration
Drawing Electron Configuration - Electron configuration chart of all elements is mentioned in the table below. We construct the periodic table by following the aufbau principle (from german, meaning “building up”). An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. The total number of electrons is the atomic number, z. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Web what is the electron configuration and orbital diagram of: Web to find the electron configuration of an element, start at hydrogen and trace across each period until your target element is reached. 1.1m views 9 years ago chemistry: Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. Shell the maximum number of electrons that can fill each is: Web by. Let’s practice in this section below. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals. Then, add or remove electrons depending on the ion's charge. At each preceding element, pay attention to the energy level and block it represents. One again we will use the example of lithium. The representative elements are those in which the distinguishing electron enter an s or p subshell. Web this chemistry video tutorial provides a basic introduction into orbital. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. 3.3m views 6 years ago new ap & general chemistry video playlist. Let’s practice in this section below. Web updated on february 01, 2021. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. At each preceding element, pay attention to the energy level and block it represents. Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Shell the maximum number of electrons that can fill each is: Under the orbital approximation, we let each electron. Electron configuration diagrams | properties of matter | chemistry | fuseschool learn the basics about. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. Electrons must occupy the lowest available shell, closest to the nucleus. We construct the periodic table by following the aufbau principle (from german, meaning. We start with a single hydrogen. Web how to draw an electron configuration diagram. It looks something like this. Electron configuration chart of all elements is mentioned in the table below. Use these steps to draw electron configuration diagrams for the first 20 elements in the periodic table. Web the electron configuration of an atom. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. Web this chemistry video tutorial provides a basic. We construct the periodic table by following the aufbau principle (from german, meaning “building up”). For example, to find the configuration for the lithium ion (li⁺), start with neutral lithium (1s²2s¹). An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. What is an electron configuration? By knowing the. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. At each preceding element, pay attention to the energy level and block it represents. Web updated on february 01, 2021. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons.. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Web the electron configuration of an atom. Web electron configurations describe where electrons are located around the nucleus of an atom. This chemistry video tutorial provides a basic. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The total number of electrons is the atomic number, z. Then, add or remove electrons depending on the ion's charge. An electron configuration shows the distribution of electrons of an atom or a molecule. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. Web the electron configuration of an element is the arrangement of its electrons in its atomic orbitals. It explains how to write the orbital diagram n. Web updated on february 01, 2021. For example, to find the configuration for the lithium ion (li⁺), start with neutral lithium (1s²2s¹). Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. The electron configuration is the standard notation used to describe the electronic structure of an atom.
Organization of Electrons in Atoms Introductory Chemistry
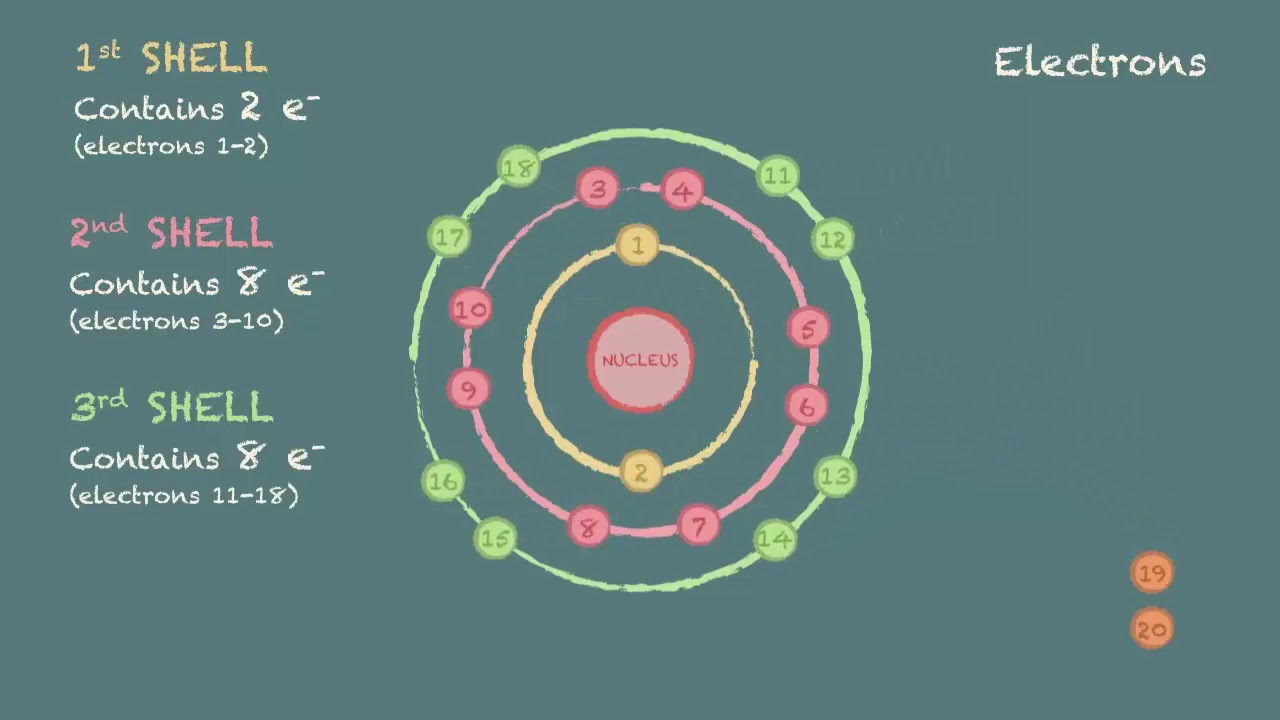
Drawing electron configuration diagrams YouTube

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts

List of Electron Configurations of Elements
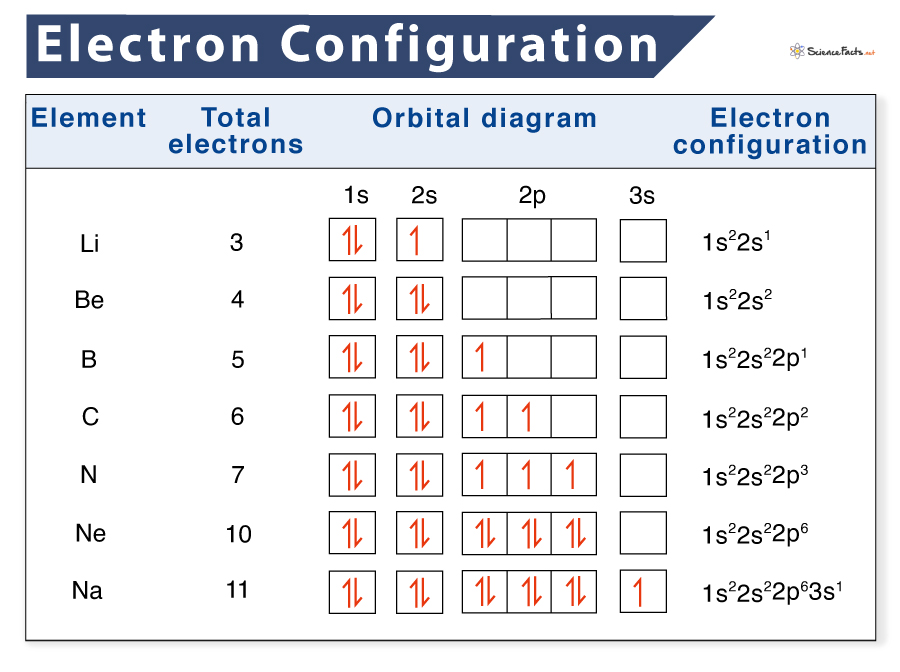
Electron Configuration Definition, Examples, Chart, and Diagram
:max_bytes(150000):strip_icc()/chlorineatom-58b602515f9b5860464c5c02.jpg)
Atom Diagrams Electron Configurations of the Elements
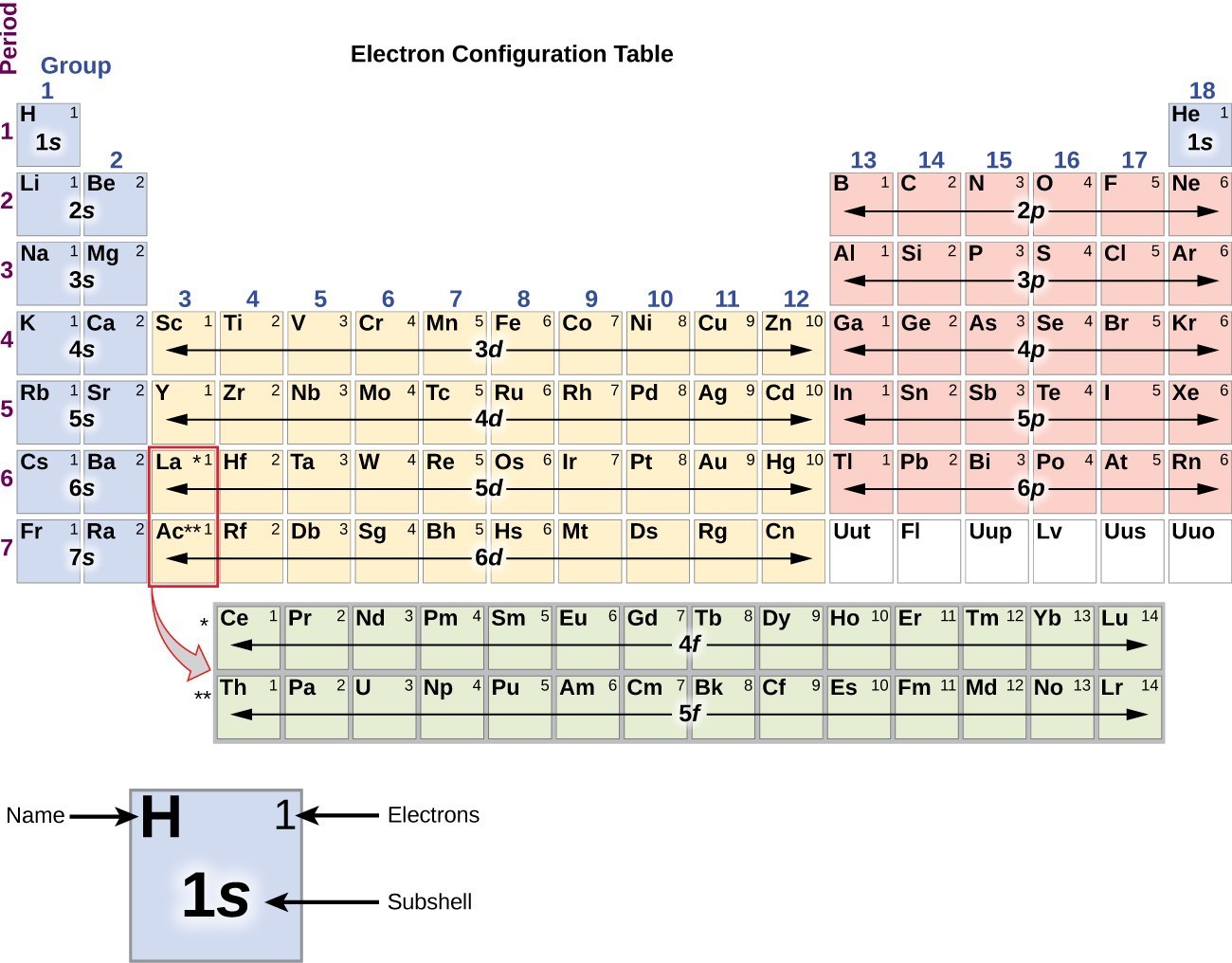
Electronic Structure of Atoms (Electron Configurations) Chemistry
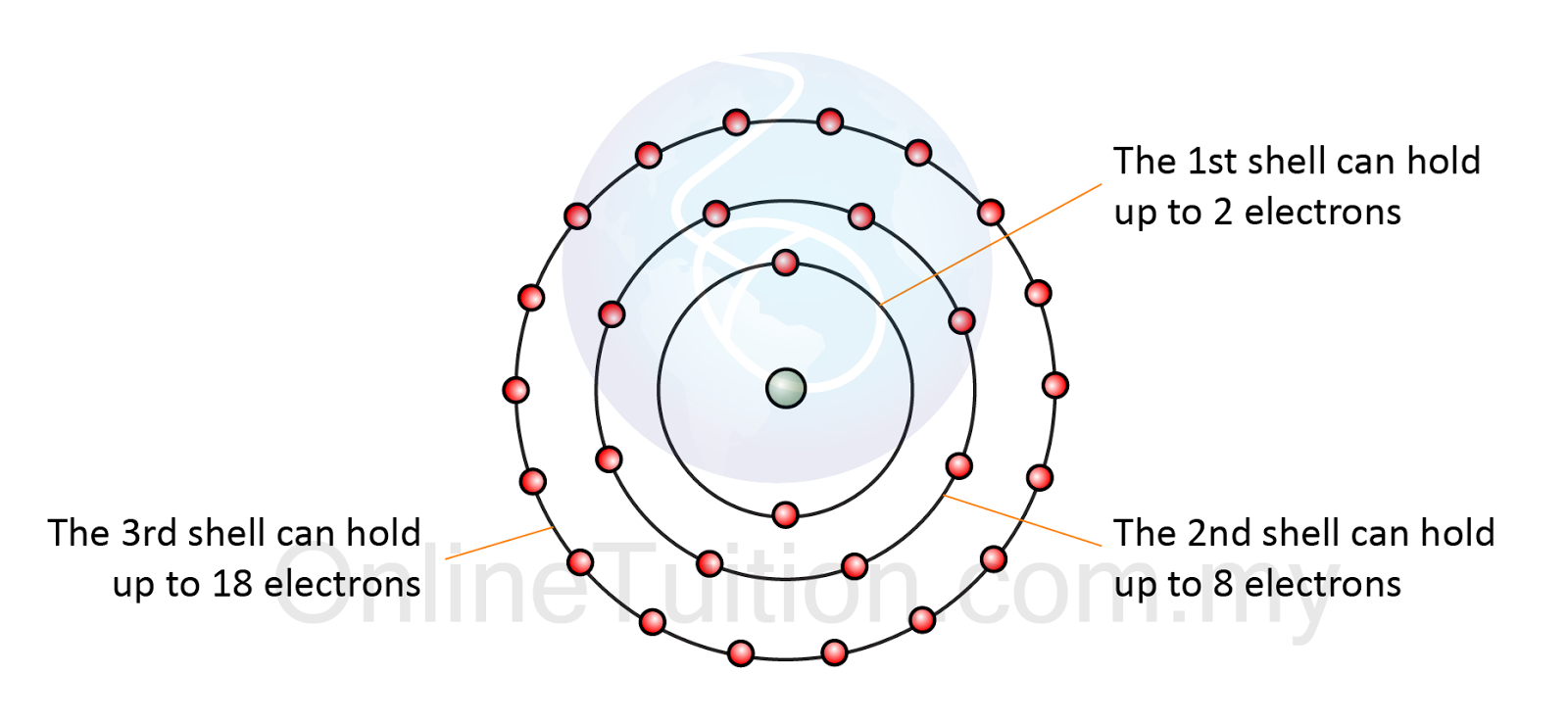
Electron Arrangement in Atom SPM Chemistry
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Electron Configuration Chart

2.2 Electron Configurations Chemistry LibreTexts
Web This Chemistry Video Tutorial Provides A Basic Introduction Into Orbital Diagrams And Electron Configuration.
Web To Find The Electron Configuration Of An Element, Start At Hydrogen And Trace Across Each Period Until Your Target Element Is Reached.
There Is A Specific Notation That Can Quickly Show You Where The Electrons Are Likely To Be Located, So Knowing This Notation Is An Essential Part Of Knowing Electron Configurations.
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Related Post: