Draw The Structure Of Butane
Draw The Structure Of Butane - Draw the structure of butane in line bond or skeleton mode. Web butane is an organic compound with the formula c4h10. If we rotate the front, (blue) carbon by 60 ° clockwise, the butane molecule is now in a staggered conformation. Web butane has two structural (also known as constitutional) isomers: Structural formulae of isomers of butane are: Let’s get started by drawing butane in the laziest way possible, with all carbons in the plane of the page; Web so if i draw a line right here, we can see there's an energy difference between our two staggered conformations. Chemspider also provides access to spectra, vendors, articles and other data sources for millions of compounds. Web we will look at the structure of butane and draw its newman projection. We use several kinds of formulas to describe organic compounds. It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. We use several kinds of formulas to describe organic compounds. This is the highest energy conformation for butane, due to what is called ‘ van der waals repulsion ’, or ‘ steric repulsion’, between the two rather bulky methyl groups.. One means the functional group is ketone. Web in this question, we are tasked with drawing the structure of butane and representing it using line. Butane is primarily used as a gasoline mixture, either alone or in a propane mixture. A) draw the complete structure of butane. The structure of butanone molecule is shown above. Web in the case of ethane, conformational changes are very subtle, but in others they are more obvious. Web below are two representations of butane in a conformation which puts the two ch 3 groups (c 1 and c 4) in the eclipsed position. Web draw electron dot structure of butane. The point of this post is to demonstrate a. However, even a simple molecule like butane can be represented a ton of different ways. Butane is a saturated hydrocarbon containing 4 carbons, with an unbranched structure. Draw the structural formula of the two isomers of butane. Web below are two representations of butane in a conformation which puts the two ch 3 groups (c 1 and c 4) in. (do not show the hydrogen atoms.) this problem has been solved! This is the highest energy conformation for butane, due to what is called ‘ van der waals repulsion ’, or ‘ steric repulsion’, between the two rather bulky methyl groups. The structures also display the total number of lone pairs present in each of the atoms that constitute the. Structural formulae of isomers of butane are: It’s four carbons in a row. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (do not show the hydrogen atoms.) this problem has been solved! Staggered and eclipsed conformations of butane. Butane is a saturated hydrocarbon containing 4 carbons, with an unbranched structure. Going from this staggered conformation up here to this eclipse conformation takes energy. Draw the structure of butane in line bond or skeleton mode. A) draw the complete structure of butane. The structure of butanone molecule is shown above. Staggered and eclipsed conformations of butane. Give the correct i u p a c name of each isomer. Hydrogen atoms and it’s the same case. Draw the structural formula of the two isomers of butane. Web chemspider is a free online database of chemical structures and properties. Web below are two representations of butane in a conformation which puts the two ch 3 groups (c 1 and c 4) in the eclipsed position. Constitutional isomers of butane (based on connectivity) We use several kinds of formulas to describe organic compounds. Hydrogen atoms and it’s the same case. Give the correct i u p a c name of. The structures also display the total number of lone pairs present in each of the atoms that constitute the molecule. A molecular formula shows only the kinds and numbers of atoms in a molecule. Staggered and eclipsed conformations of butane. This is the highest energy conformation for butane, due to what is called ‘ van der waals repulsion ’, or. Going from this staggered conformation up here to this eclipse conformation takes energy. Not much more to it than that. However, even a simple molecule like butane can be represented a ton of different ways. The structure of butanone molecule is shown above. Web below are two representations of butane in a conformation which puts the two ch 3 groups (c 1 and c 4) in the eclipsed position. One means the functional group is ketone. Let’s get started by drawing butane in the laziest way possible, with all carbons in the plane of the page; The hydrocarbon butane has a larger and more complex set of conformations associated with its constitution than does ethane. Web we will look at the structure of butane and draw its newman projection. Web so if i draw a line right here, we can see there's an energy difference between our two staggered conformations. Give the correct i u p a c name of each isomer. The structures also display the total number of lone pairs present in each of the atoms that constitute the molecule. You'll get a detailed solution from a subject matter expert. Web in the case of ethane, conformational changes are very subtle, but in others they are more obvious. Butane is a saturated hydrocarbon containing 4 carbons, with an unbranched structure. Draw the structure of butane in line bond or skeleton mode.
Butane Molecule Image Royalty Free Vector Image
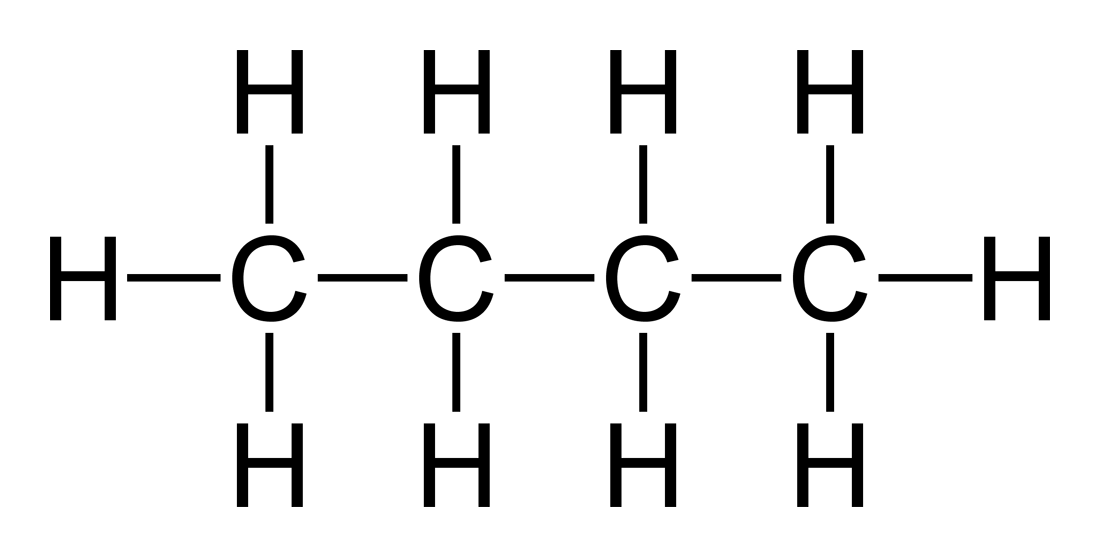
Shapes of Molecules Chemistry Coach
[Solved] Draw butane (C 4 H 10 ). What class of organic molecule is
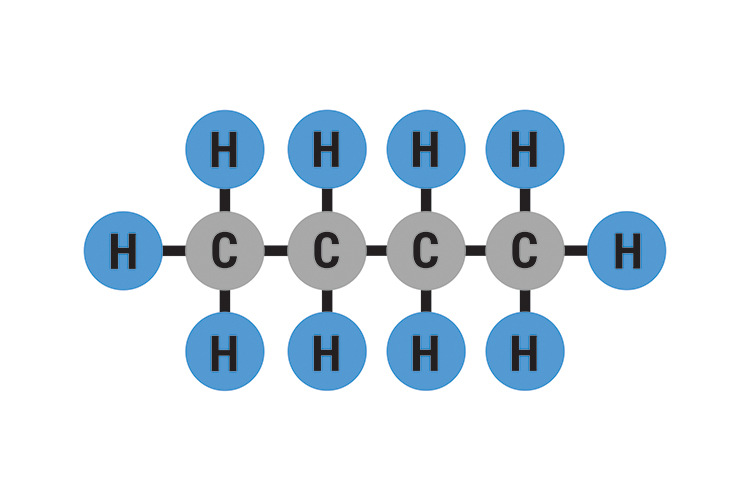
The molecular structure of Butane and formula structure

The illustration of the butane structural formula Stock Vector Image

Butane Molecular Geometry Hybridization Molecular Weight
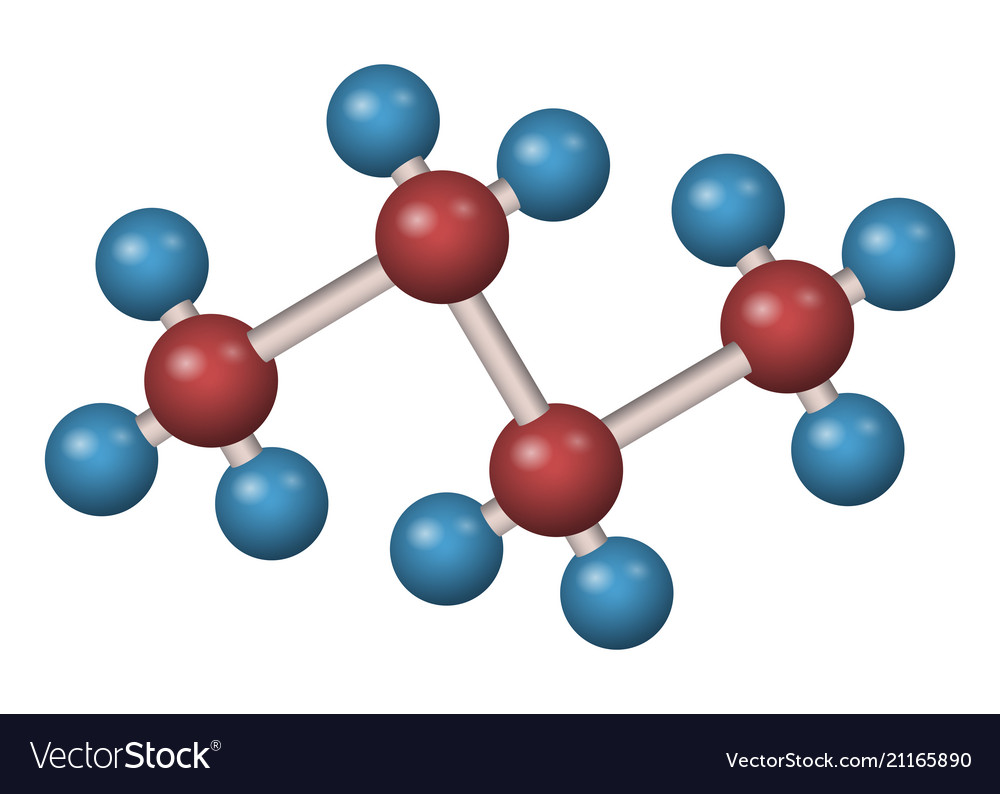
Butane molecule is a 3d formula Royalty Free Vector Image

Molecular model of Butane Stock Photo Alamy
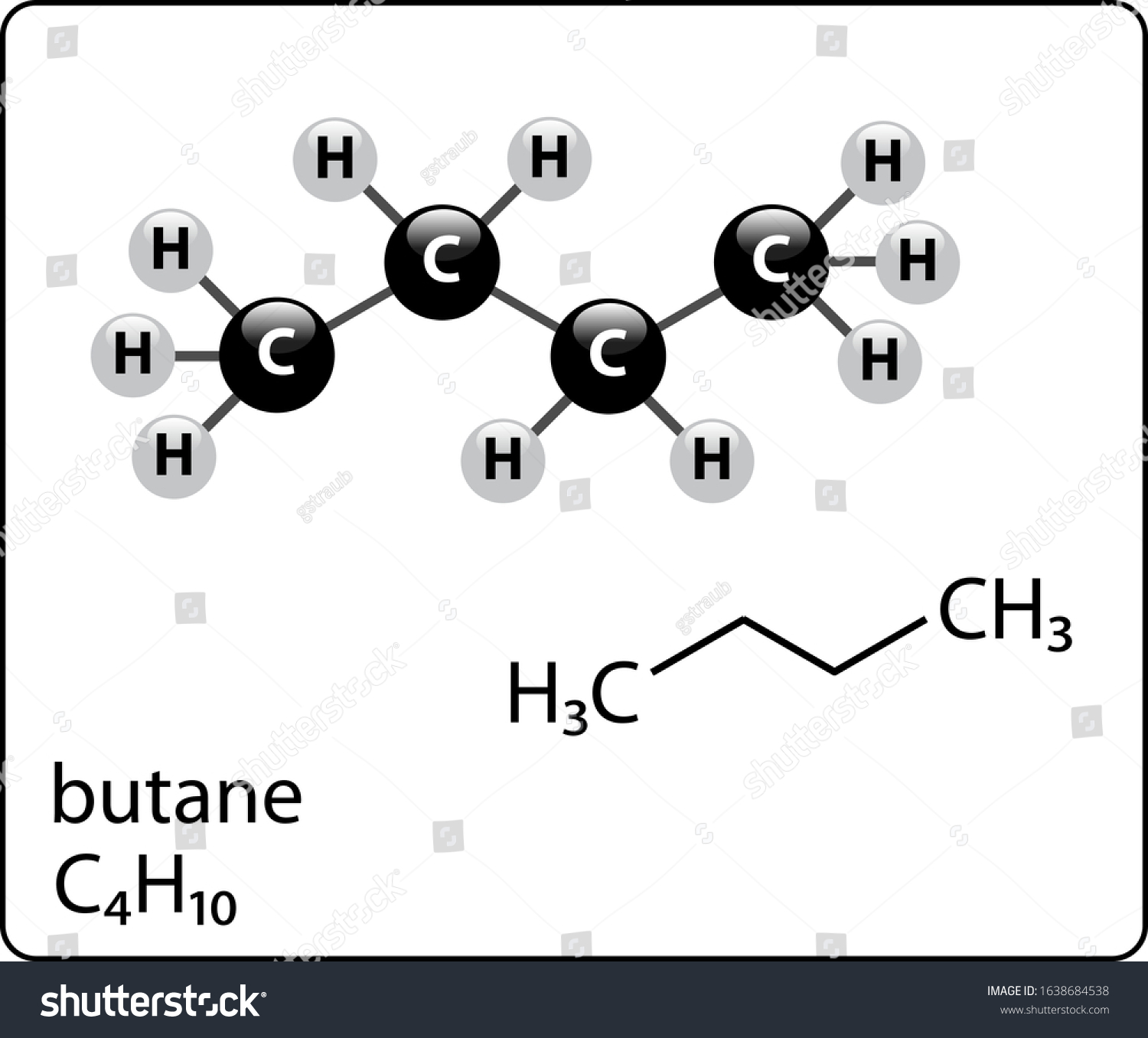
Butane Molecule Structure Ball Stick Stock Vector (Royalty Free

Draw electron dot structure of butane.
Structural Formulae Of Isomers Of Butane Are:
If We Rotate The Front, (Blue) Carbon By 60 ° Clockwise, The Butane Molecule Is Now In A Staggered Conformation.
It Is Also Used As A Feedstock For Ethylene And Butadiene Production.
It Defines The Nature Of The Bond And Position Of Atoms Of The Molecule Which Are Connected In The Molecule.
Related Post: