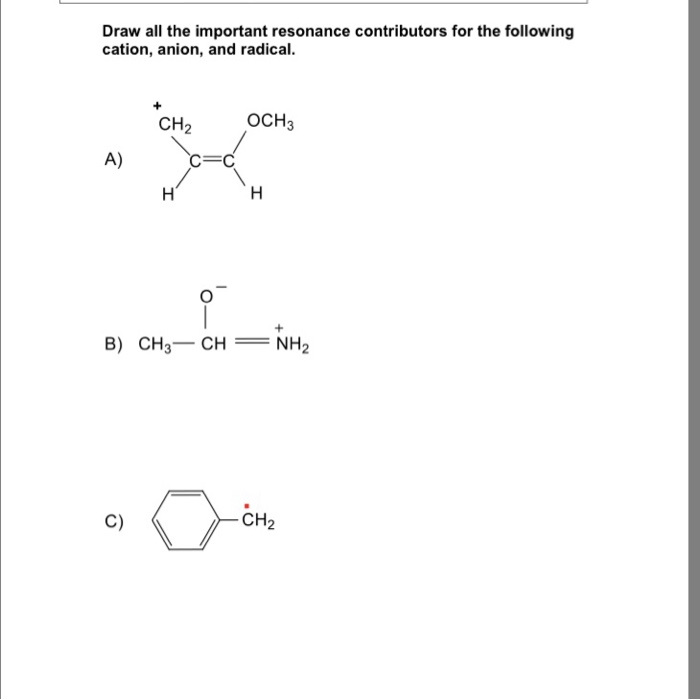
Draw The Resonance Contributors For
Draw The Resonance Contributors For - According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the. We just need a graphical tool to do it. Web draw the lewis structure & resonance for the molecule (using solid lines for bonds). Draw the resonance contributors for. This video solution was recommended by our tutors as helpful for the problem above. Part d draw the resonance contributors for draw all possible resonance structures by copying the skeleton shown. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Where there can be a double or triple bond, draw a dotted. If either structure were correct, benzene would consist of alternating long single bonds and short double bonds. The structure is at all times a single resonance hybrid of all the structures. We can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). Edit bonds and charges to complete each resonance structure. 2) draw four additional resonance contributors for the molecule below. Explain why your contributor is. Explain why your contributor is the major one. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. Resonance structures and major contributor. Web why atoms react to form molecules? Draw resonance contributors for. 29 views 6 years ago fundamentals of organic chemistry. According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the. This video solution was recommended by our tutors as helpful for the problem above. Web why atoms react to form molecules? Follow the rules to determine the major. Learn how draw the most stable resonance structures and determine the. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Resonance contributors and the resonance hybrid. Draw the important resonance contributors for the sigma complex shown here: Draw the resonance contributors for. Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. A review of general chemistry resonance structures. Draw all possible resonance structures by copying the skeleton shown. Two good lewis structures for benzene exist that differ only in their placement of double bonds. Web draw the major resonance contributor. Web these two drawings are an example of what is referred to in organic chemistry as resonance contributors: Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Web draw resonance forms and predict the relative contribution of each resonance form to the overall structure of the compound or ion. 2) draw four additional resonance contributors. The classical example of resonance is benzene, c6h6. Web when you draw resonance structures in your head, think about what that means for the hybrid, and how the resonance structures would contribute to the overall hybrid. Web these two drawings are an example of what is referred to in organic chemistry as resonance contributors: That one will be the major. Web we just have to draw the resonance contributors in some order that makes sense. Web in cases in which more than one reasonable (plausible) lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Explain why your contributor is the major one. Web introducing curved arrows, a tool for showing the movement. Web this organic chemistry video tutorial explains how to draw resonance structures, how to identify the major resonance contributor, and how to draw the resonance hybrid. Web here, we will focus on how to draw resonance structures (or resonance contributors) for organic chemistry species and how to compare the relative stabilities between the structures. Resonance contributors and the resonance hybrid.. 29 views 6 years ago fundamentals of organic chemistry. The general approach is described below: Draw the important resonance contributors for the sigma complex shown here: Web when you draw resonance structures in your head, think about what that means for the hybrid, and how the resonance structures would contribute to the overall hybrid. Explain why your contributor is the. Web more resonance contributors can be drawn in which negative charge is delocalized to three other atoms on the molecule. Draw the resonance structure (s). Web each lewis structure that contributes to the resonance hybrid is a resonance structure. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). 2) draw four additional resonance contributors for the molecule below. Web in cases in which more than one reasonable (plausible) lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. A review of general chemistry resonance structures. According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the. Draw resonance contributors for each of the following species and rank them in order of decreasing contribution to the resonance hybrid. Draw the important resonance contributors for the sigma complex shown here: Web draw the major resonance contributor of the structure below. Web when you draw resonance structures in your head, think about what that means for the hybrid, and how the resonance structures would contribute to the overall hybrid. Two good lewis structures for benzene exist that differ only in their placement of double bonds. That one will be the major contributor. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an alkane is >2), chemists expected their structural formulas would contain a large number of double or triple bonds. The structure is at all times a single resonance hybrid of all the structures.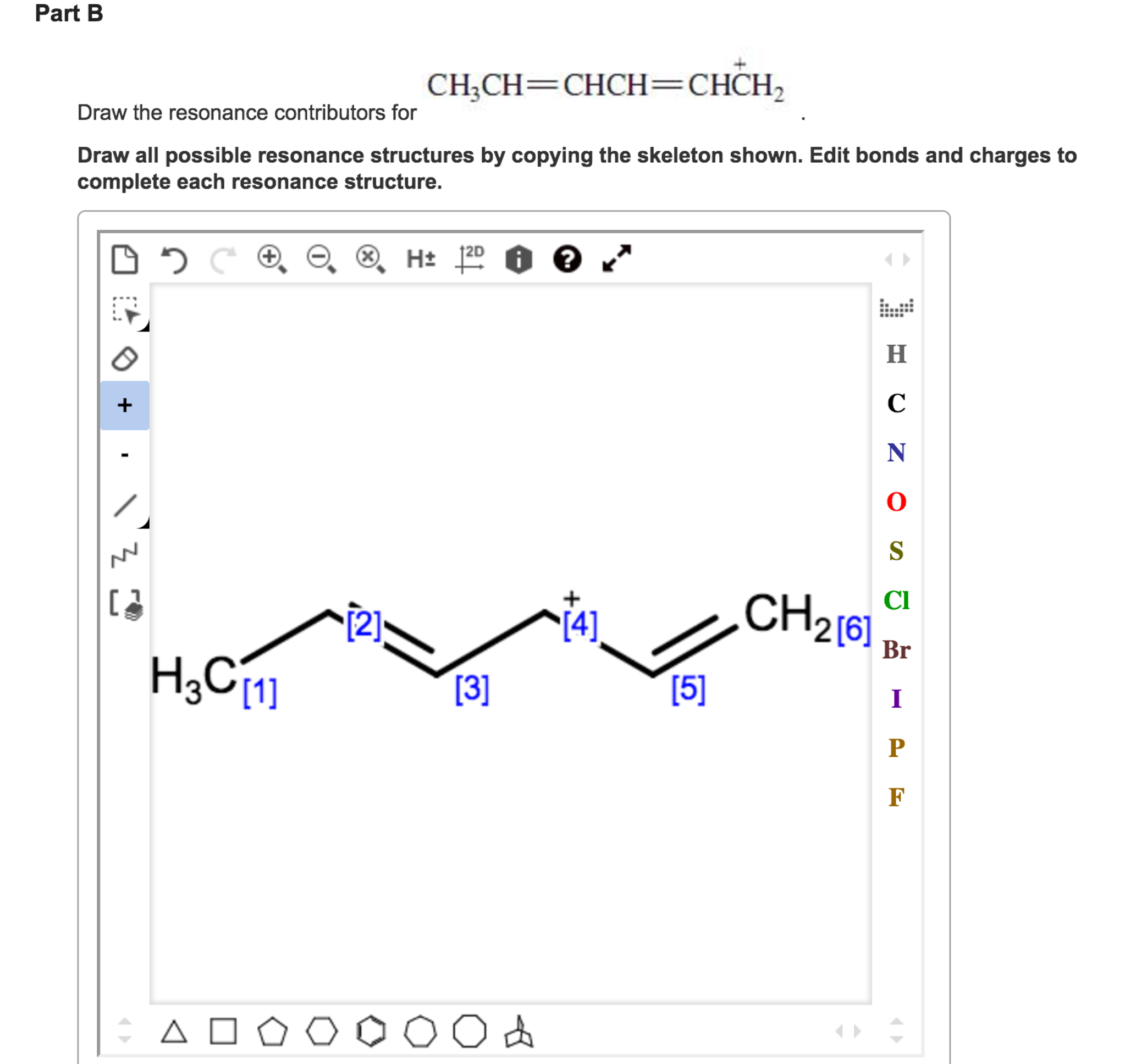
Solved Draw the resonance contributors for CH_CH=CHCH=CHCH_2
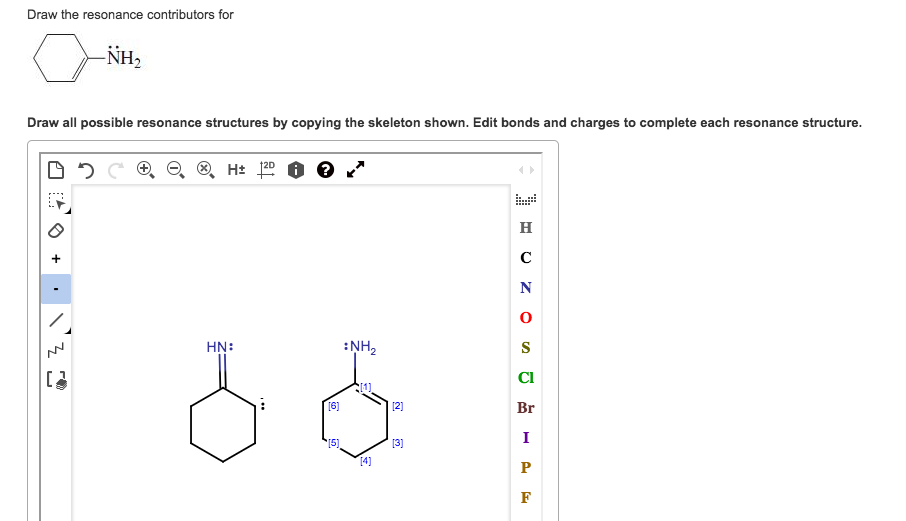
Solved Draw the resonance contributors for NH2 Draw all
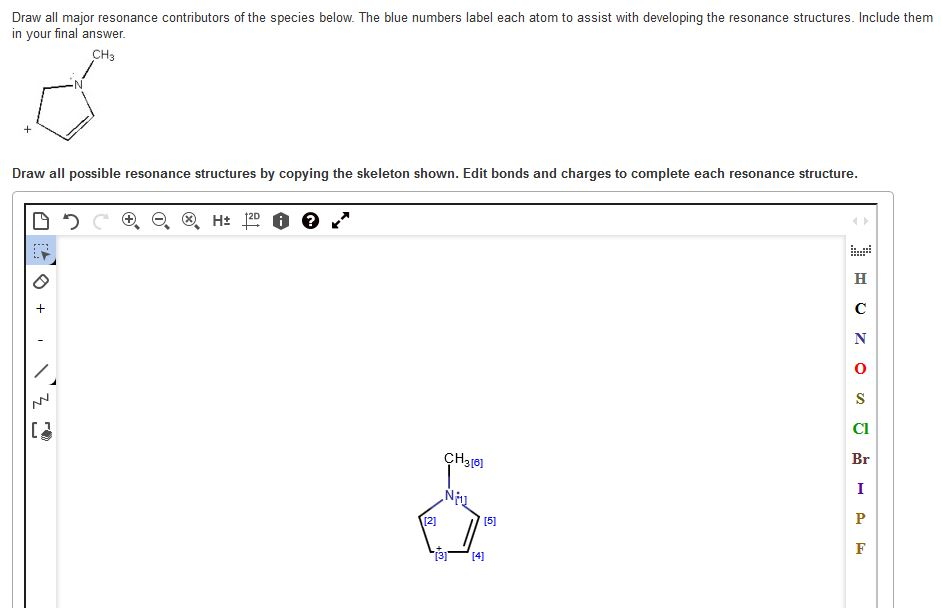
Solved Draw all major resonance contributors of the species
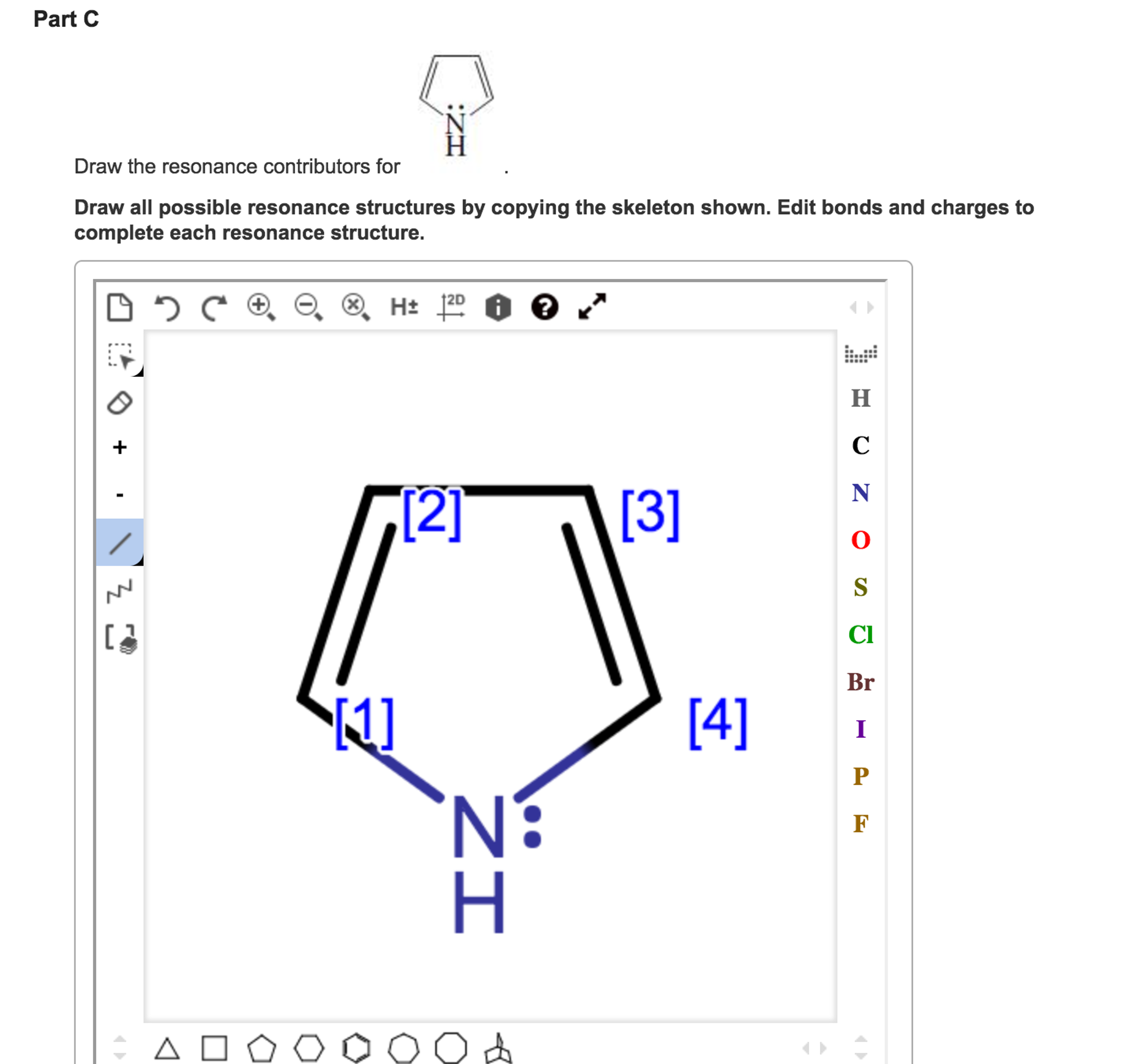
Solved Draw the resonance contributors for. Draw all
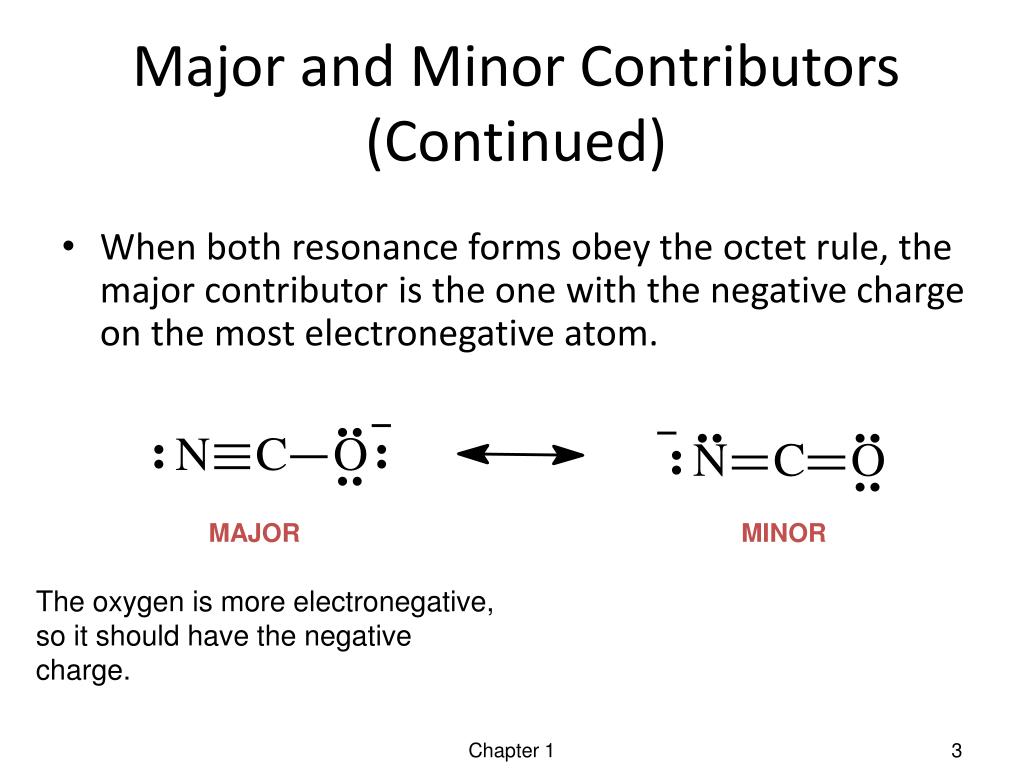
PPT Resonance Forms PowerPoint Presentation, free download ID1981144
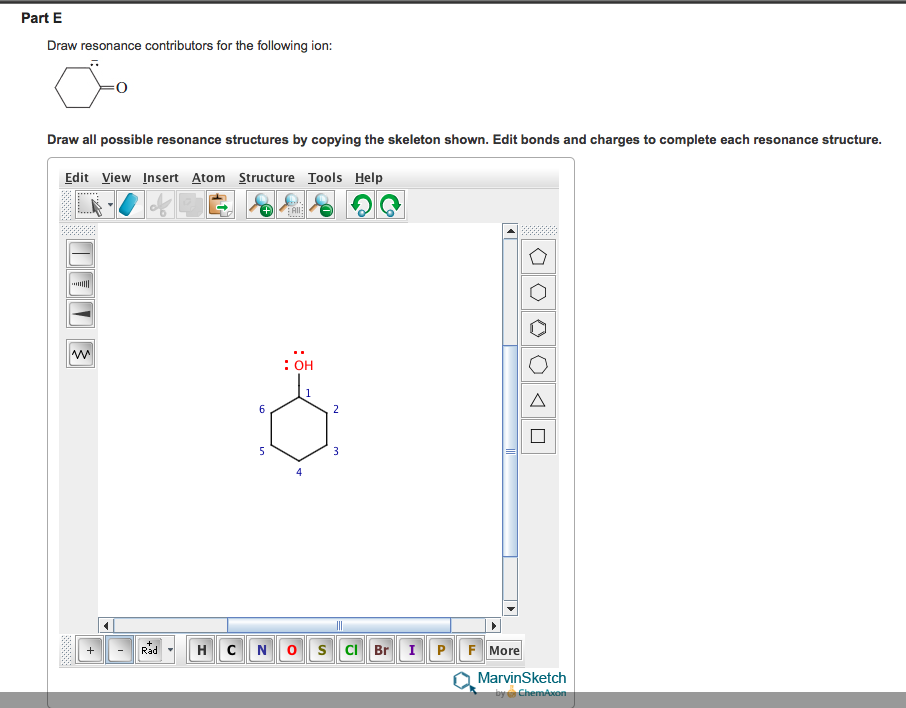
Solved Draw resonance contributors for the following ion. I
Solved Draw all the important resonance contributors for the
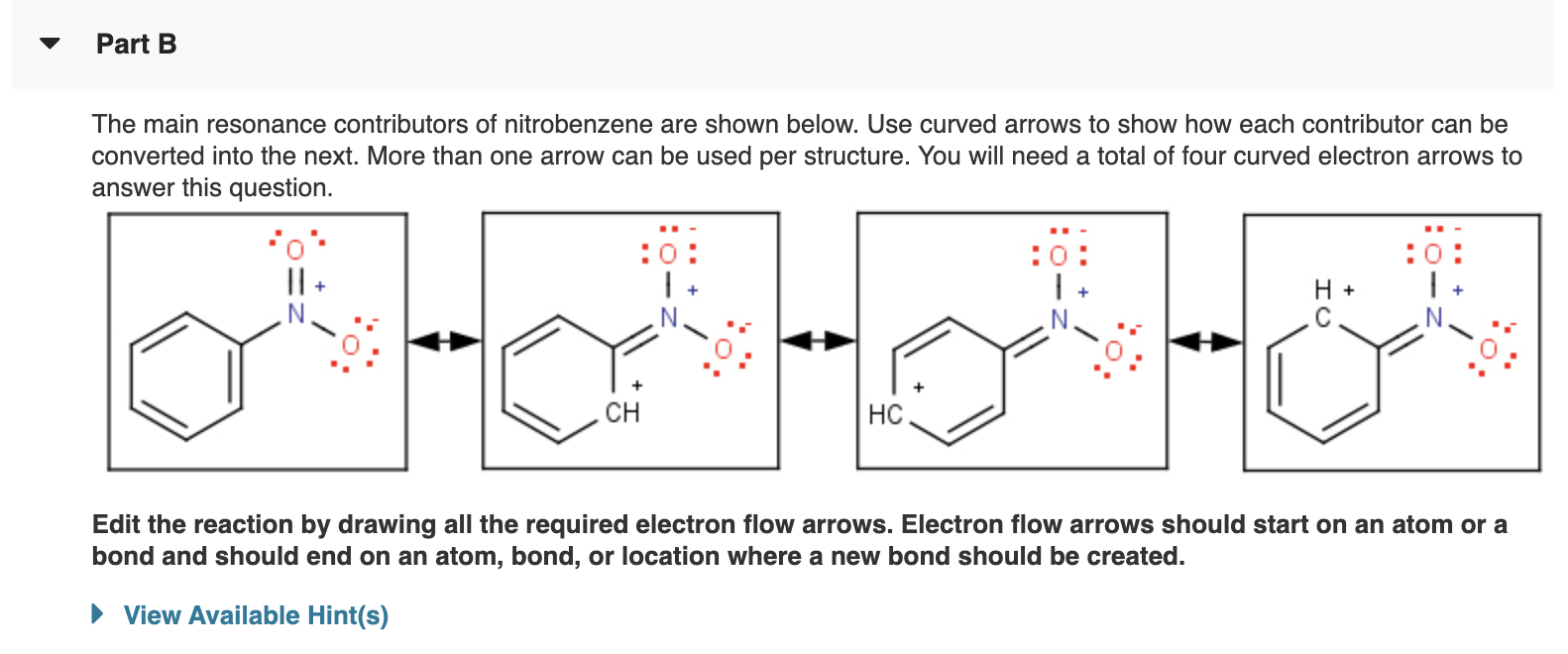
Solved Part B The main resonance contributors of

How to Draw Resonance Contributors MCC Organic Chemistry
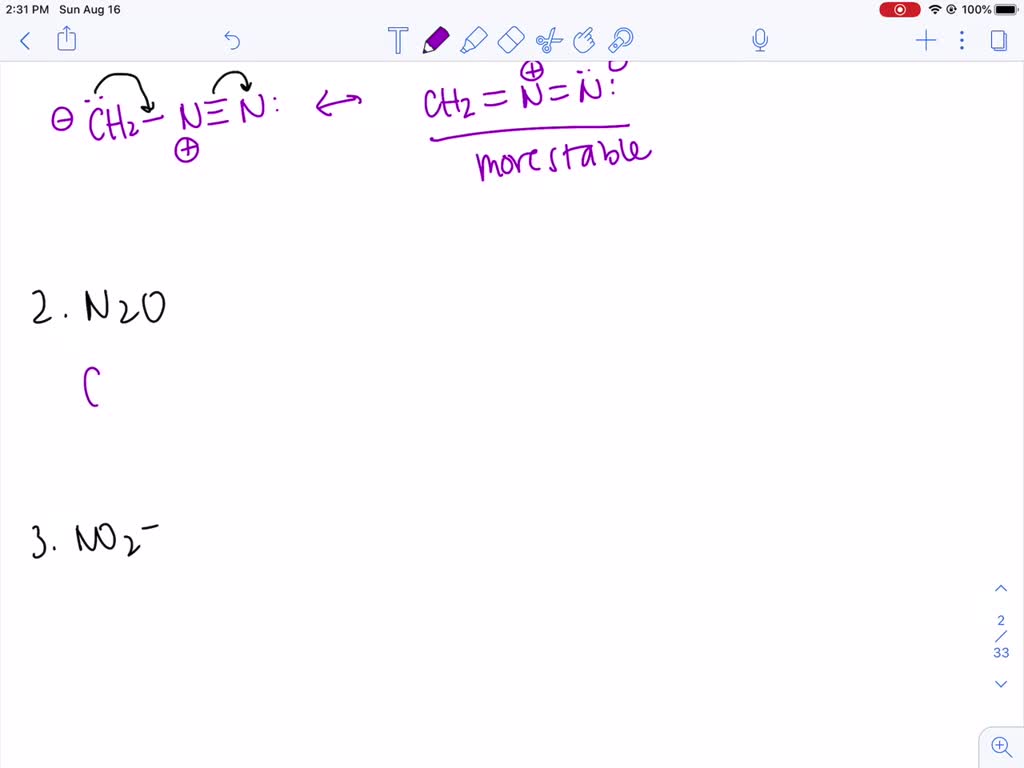
SOLVEDa. Draw resonance contributors for the following species
All The Others Will Be Minor.
Web 1) For The Following Resonance Structures Please Rank Them In Order Of Stability.
Web Draw Resonance Forms And Predict The Relative Contribution Of Each Resonance Form To The Overall Structure Of The Compound Or Ion.
Web Here, We Will Focus On How To Draw Resonance Structures (Or Resonance Contributors) For Organic Chemistry Species And How To Compare The Relative Stabilities Between The Structures.
Related Post: