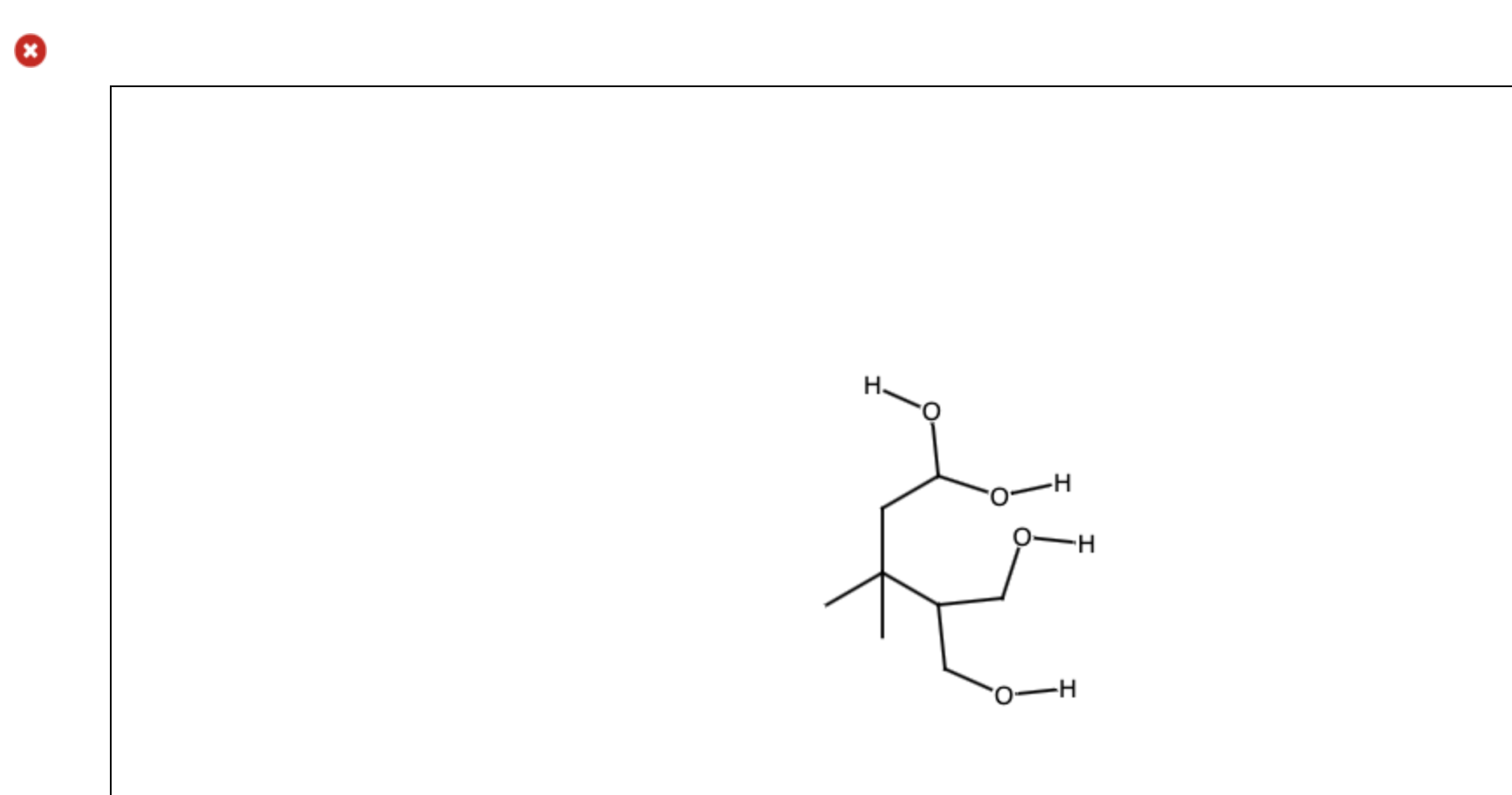
Draw The Organic Product For The Following Acidcatalyzed Hydrolysis Reaction
Draw The Organic Product For The Following Acidcatalyzed Hydrolysis Reaction - Web transfer of a proton (step 3, arrows e and f) followed by 1,2 elimination of ammonia (step 4, arrows g and h) lead to an oxonium ion, which is then deprotonated to give the. Web biological amide hydrolysis, as in the hydrolysis of peptides and proteins, is catalyzed by the proteolytic enzymes. These reactions will be discussed in chapter 25. Acidic hydrolysis of an ester gives the product of a carboxylic acid and an alcohol. H2so4, h20 i i h 5th attempt x incorrect @ see page 897 incorrect. The nucleophilic water reacts with. Web in this video, we're talking about the reverse reaction; You should now be comfortable enough to draw the mechanism for both reactions, but. Web if you use strong acid or a strong base and you heat things up for several hours, you can hydrolyze amides. This problem has been solved! These reactions will be discussed in chapter 25. H2so4, h20 i i h 5th attempt x incorrect @ see page 897 incorrect. Acidic hydrolysis of an ester gives the product of a carboxylic acid and an alcohol. You should now be comfortable enough to draw the mechanism for both reactions, but. Web in this video, we're talking about the reverse. Web biological amide hydrolysis, as in the hydrolysis of peptides and proteins, is catalyzed by the proteolytic enzymes. So if we increase the concentration of water, that would shift the equilibrium. Web transfer of a proton (step 3, arrows e and f) followed by 1,2 elimination of ammonia (step 4, arrows g and h) lead to an oxonium ion, which. Web predict the products, specify the reagents, and discern most efficient reaction for hydration of alkenes (acid catalyzed hydration; You should now be comfortable enough to draw the mechanism for both reactions, but. Web in the presence of an acid catalyst (h2so4) and water (h2o), the ester will undergo hydrolysis to form a carboxylic acid (rcooh) and an alcohol (r'oh).. So if we increase the concentration of water, that would shift the equilibrium. If we take a look at this amide right here, we can break this bond. H2so4, h20 i i h 5th attempt x incorrect @ see page 897 incorrect. Acidic hydrolysis of an ester gives the product of a carboxylic acid and an alcohol. Web biological amide. Web transfer of a proton (step 3, arrows e and f) followed by 1,2 elimination of ammonia (step 4, arrows g and h) lead to an oxonium ion, which is then deprotonated to give the. The nucleophilic water reacts with. Acidic hydrolysis of an ester gives the product of a carboxylic acid and an alcohol. Web the mechanism for the. Web if you use strong acid or a strong base and you heat things up for several hours, you can hydrolyze amides. H2so4, h20 i i h 5th attempt x incorrect @ see page 897 incorrect. Web biological amide hydrolysis, as in the hydrolysis of peptides and proteins, is catalyzed by the proteolytic enzymes. So if we increase the concentration. Web in the presence of an acid catalyst (h2so4) and water (h2o), the ester will undergo hydrolysis to form a carboxylic acid (rcooh) and an alcohol (r'oh). So if we increase the concentration of water, that would shift the equilibrium. We're going to talk about ester hydrolysis. Web if you use strong acid or a strong base and you heat. These reactions will be discussed in chapter 25. Web this page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as hydrochloric acid or sulfuric acid) acting as the. The nucleophilic water reacts with. This problem has been solved! H2so4, h20 i i h 5th attempt x incorrect @ see. You should now be comfortable enough to draw the mechanism for both reactions, but. The nucleophilic water reacts with. Web biological amide hydrolysis, as in the hydrolysis of peptides and proteins, is catalyzed by the proteolytic enzymes. Web this page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as. Web this page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as hydrochloric acid or sulfuric acid) acting as the. Web the mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Web biological amide hydrolysis, as in. We're going to talk about ester hydrolysis. If we take a look at this amide right here, we can break this bond. So if we increase the concentration of water, that would shift the equilibrium. Acidic hydrolysis of an ester gives the product of a carboxylic acid and an alcohol. Web in the presence of an acid catalyst (h2so4) and water (h2o), the ester will undergo hydrolysis to form a carboxylic acid (rcooh) and an alcohol (r'oh). Web predict the products, specify the reagents, and discern most efficient reaction for hydration of alkenes (acid catalyzed hydration; You should now be comfortable enough to draw the mechanism for both reactions, but. Web if you use strong acid or a strong base and you heat things up for several hours, you can hydrolyze amides. Web transfer of a proton (step 3, arrows e and f) followed by 1,2 elimination of ammonia (step 4, arrows g and h) lead to an oxonium ion, which is then deprotonated to give the. Web in this video, we're talking about the reverse reaction; These reactions will be discussed in chapter 25. Web biological amide hydrolysis, as in the hydrolysis of peptides and proteins, is catalyzed by the proteolytic enzymes. Web this page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as hydrochloric acid or sulfuric acid) acting as the.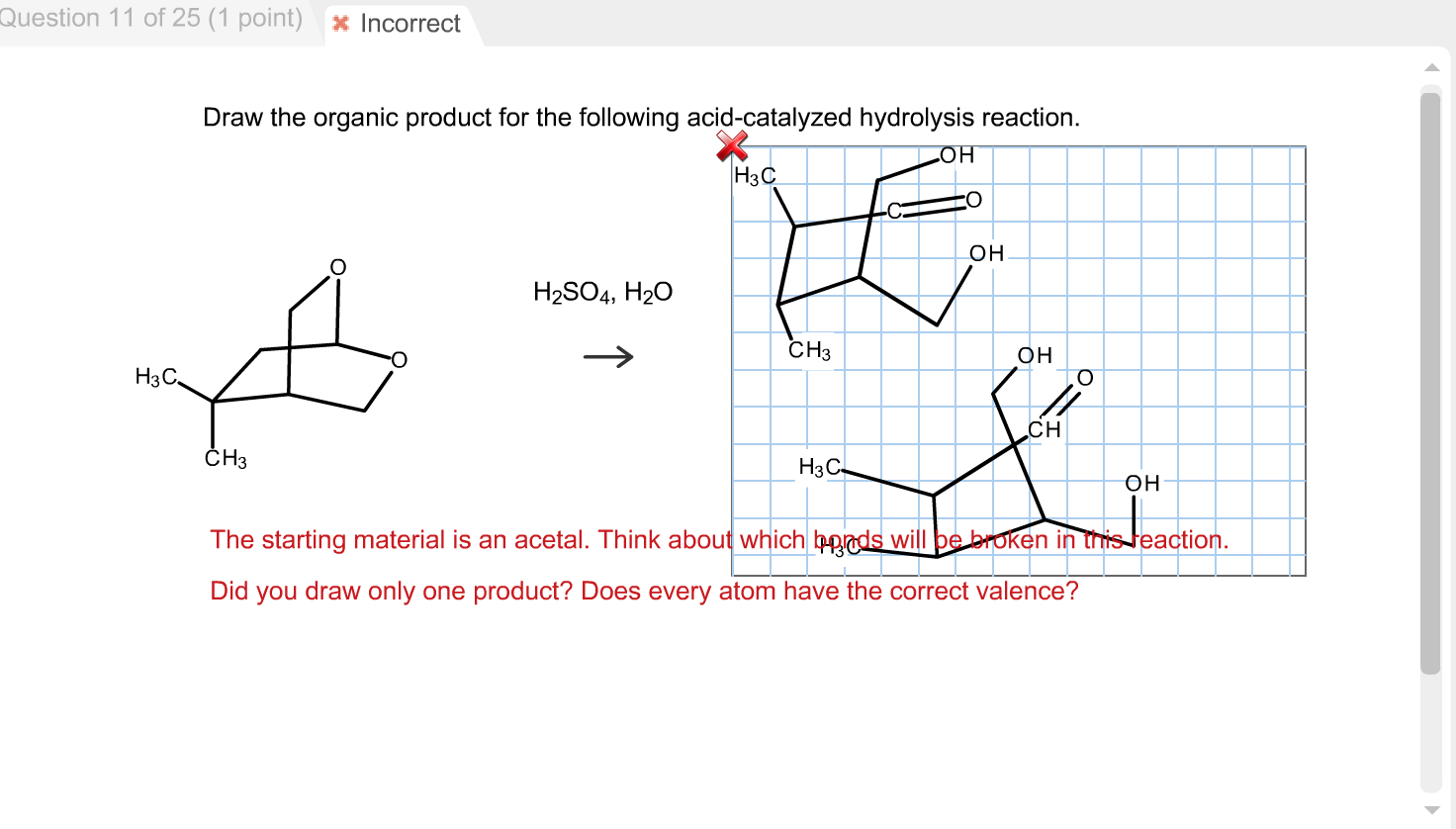
Solved Draw The Organic Product For The Following Acidca...
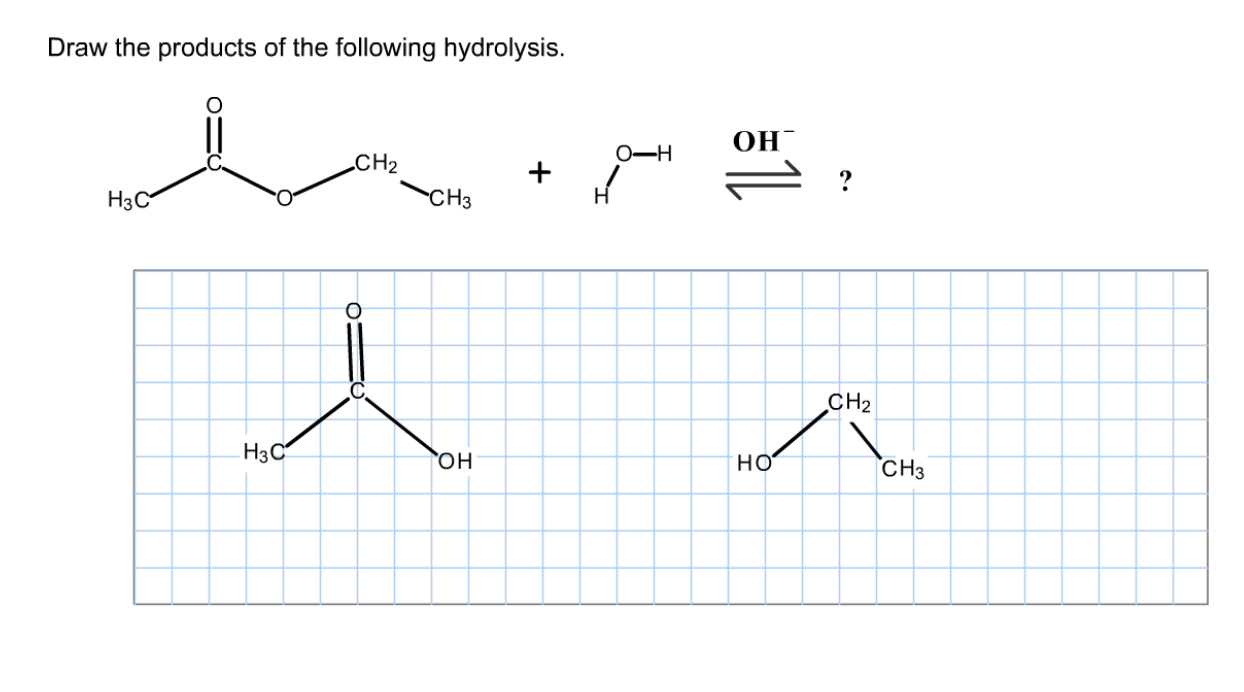
Solved Draw the products of the following hydrolysis
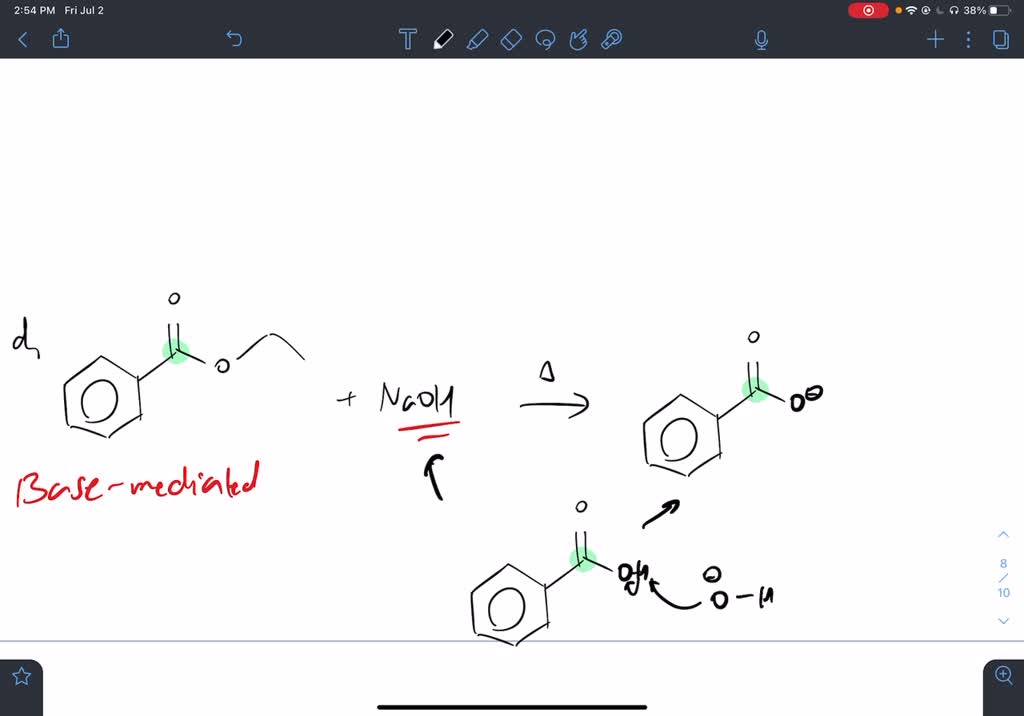
SOLVED Draw the condensed structural formulas of the products from the

2022 UPDATED!!! Draw the major organic product from the following
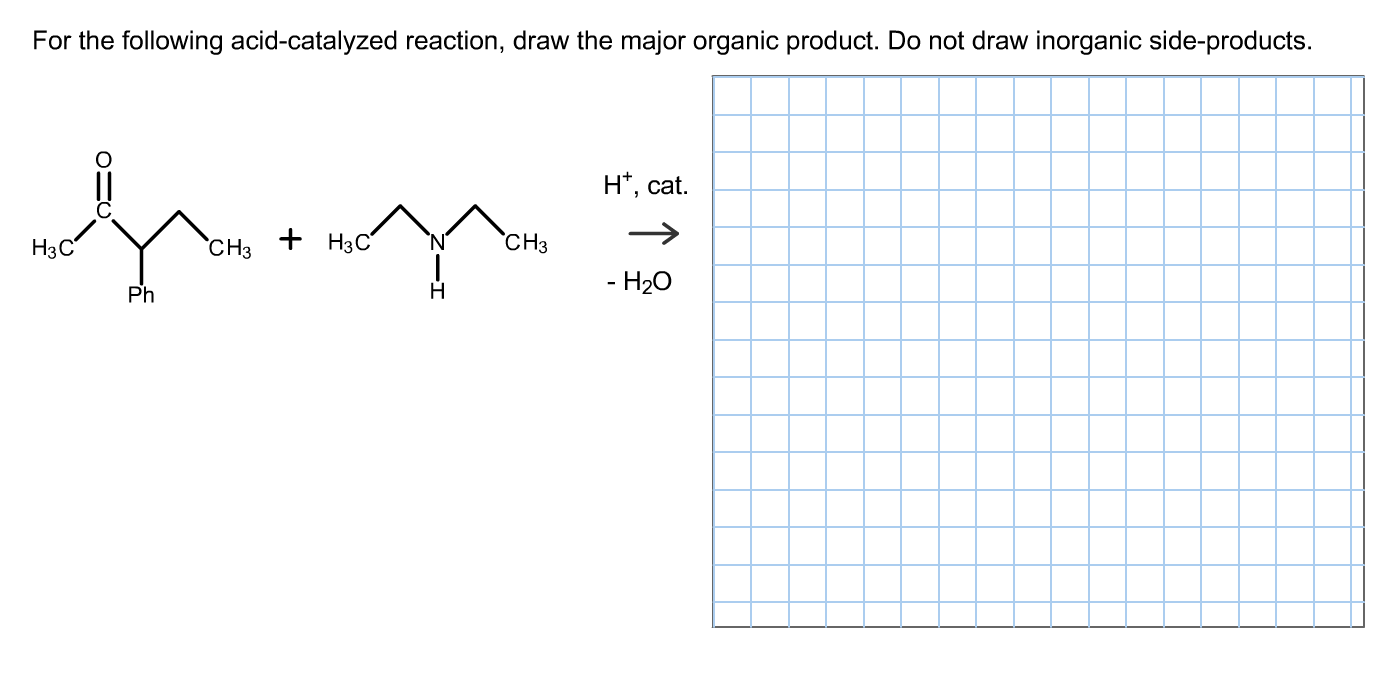
Solved For The Following Acidcatalyzed Reaction, Draw Th...
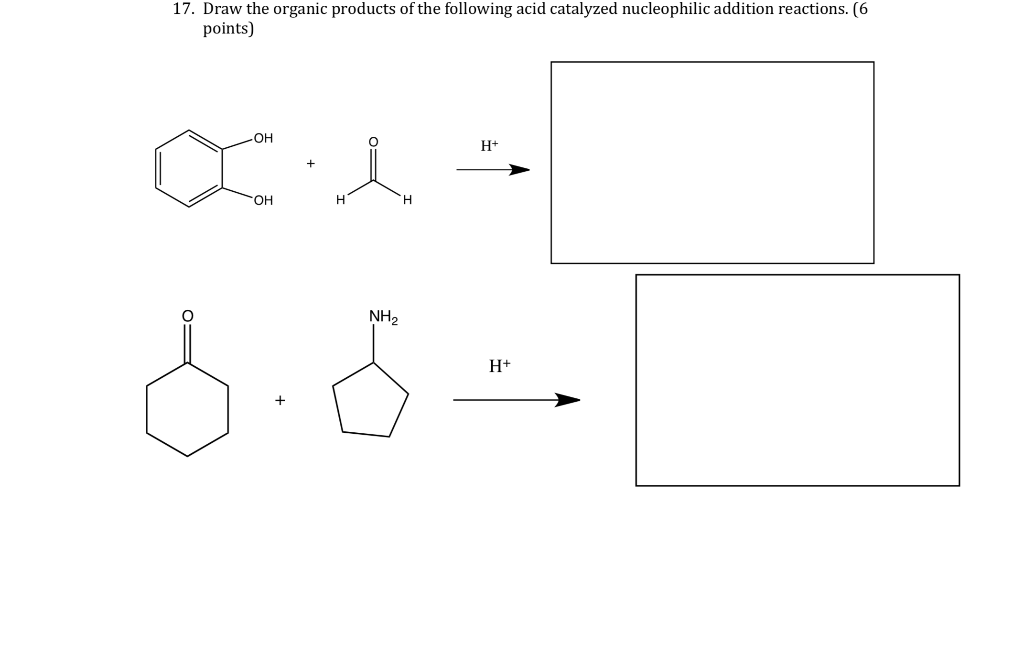
Solved 17. Draw the organic products of the following acid

Mechanism of AcidCatalyzed Hydrolysis of An Ester with a Tertiary
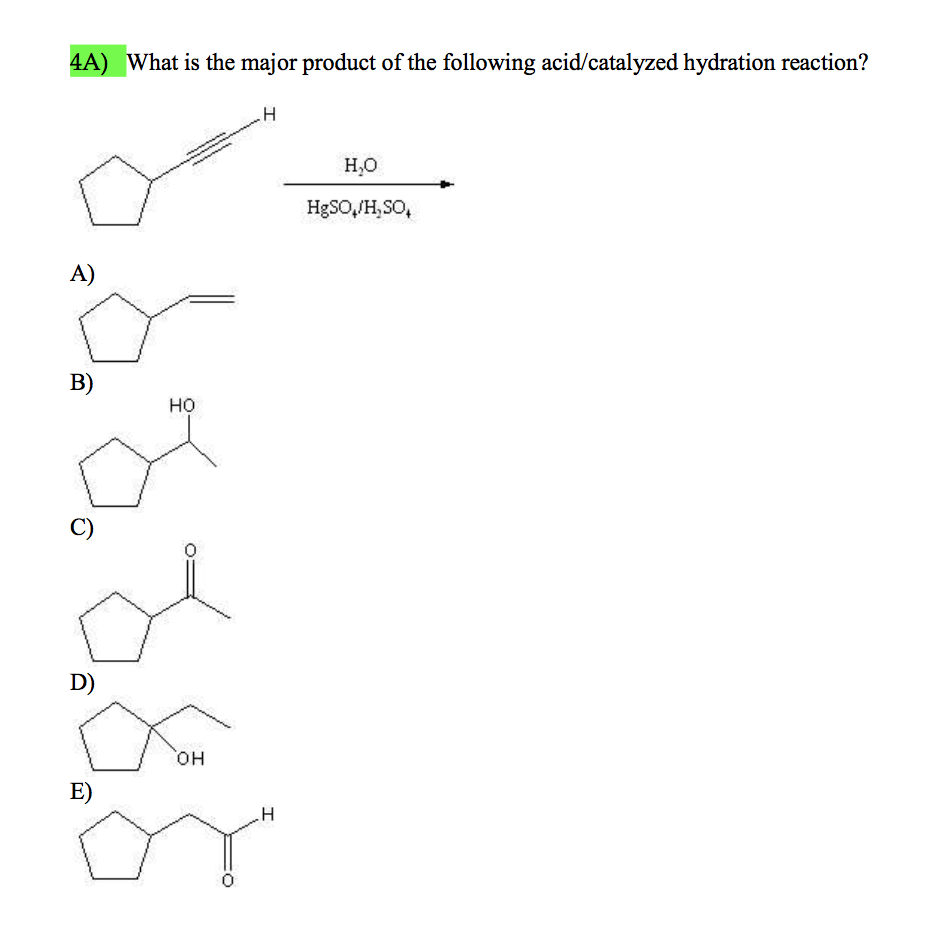
Solved What is the major product of the following
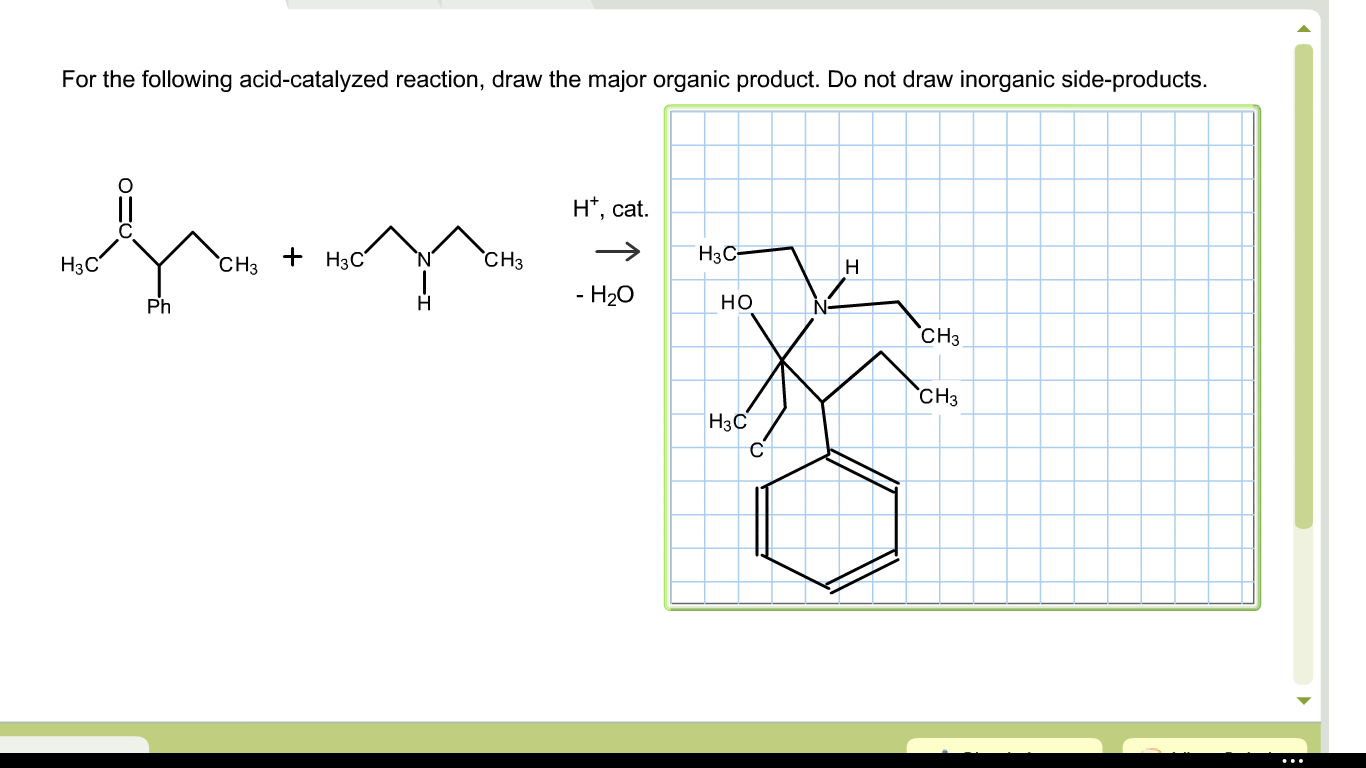
Solved For The Following Acidcatalyzed Reaction, Draw Th...
Solved 01 Question (3 points) Draw the organic product for
Web The Mechanism For The Acid Catalyzed Hydrolysis Reaction Begins With Protonation Of The Carbonyl Oxygen To Increase The Reactivity Of The Ester.
H2So4, H20 I I H 5Th Attempt X Incorrect @ See Page 897 Incorrect.
This Problem Has Been Solved!
The Nucleophilic Water Reacts With.
Related Post: