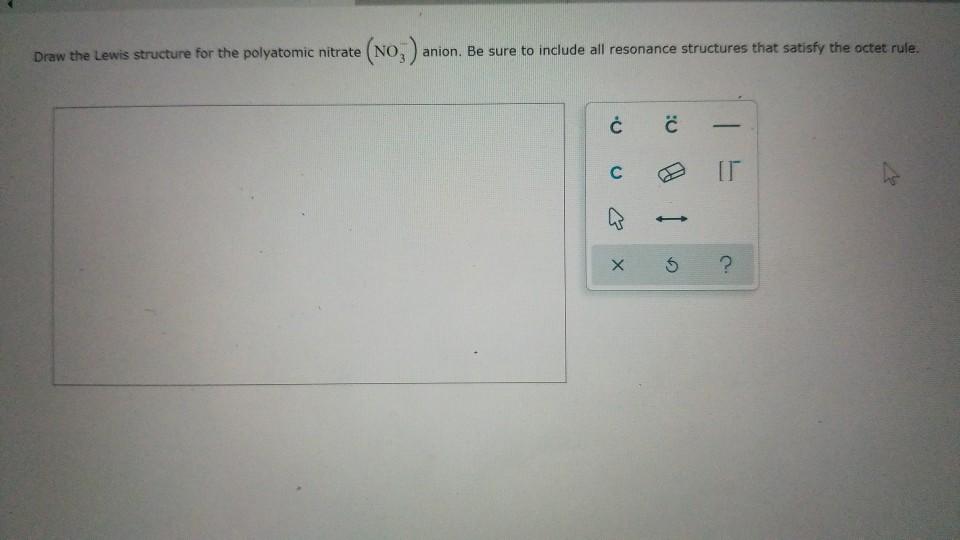
Draw The Lewis Structure For The Polyatomic Nitrate
Draw The Lewis Structure For The Polyatomic Nitrate - Here’s how to approach this question. For the positive polyatomic ammonium, we look at the three bonding sites for nitrogen and the four possible bonding sites on the four hydrogen atoms. When drawing these kinds of. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Lewis structures show all of the valence electrons in an atom or molecule. Web to draw lewis structures for molecules and polyatomic ions with one central atom. The valence electrons are the electrons in the. These structures are resonance structures that represent a single nitrate ion. A lewis structure is a way to show how atoms share electrons when they form a molecule. 46k views 3 years ago. Resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. A lewis structure is a way to show how atoms share electrons when they form a molecule. Polyatomic ions have defined formulas, names, and charges that. You'll get a detailed solution from. In the ion no3, there is 1 atom of nitrogen and 3 atoms of oxygen. Draw the lewis structure for the polyatomic nitrate no−3 anion. Contrary to his other lewis structures for nitrate, he didn't add the valence electrons around the oxygen atoms. Resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding. Finally, draw two additional resonance structures; Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. Draw the lewis structure for the polyatomic nitrate (no3−)anion. It also has one negative charge. Here’s how to approach this question. Using arguments based on formal charges, explain why the most likely. Lewis structures show all of the valence electrons in an atom or molecule. In this video, we will go through how to draw lewis structures for polyatomic ion in five easy steps. Polyatomic ions have defined formulas, names, and charges that. Three of the hydrogen bond covalently to the. Be sure to include all resonance structures that satisfy the octet rule. Web draw at least one other lewis structure for the nitrate ion that is not plausible based on formal charges. What molecular geometry would you assign to the nitrate ion? Be sure to include all resonance structures that satisfy the octet rule. Contrary to his other lewis structures. Web there are three lewis structures for the nitrate polyatomic ion. A lewis structure is a way to show how atoms share electrons when they form a molecule. The valence electrons are the electrons in the. Finally, draw two additional resonance structures; In this video, we will go through how to draw lewis structures for polyatomic ion in five easy. 46k views 3 years ago. Contrary to his other lewis structures for nitrate, he didn't add the valence electrons around the oxygen atoms. When drawing these kinds of. 152k views 3 years ago. N = 5 (from nitrogen) + 6 (from oxygen) = 11 + 18 = 29. Polyatomic ions have defined formulas, names, and charges that. 4k views 5 years ago covalent bonding. At least two lewis structures can be drawn for bcl 3. Indicate any bond dipoles and the overall dipole moment fot the anion. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Here’s the best way to solve it. Polyatomic ions have defined formulas, names, and charges that. Names, formula, and charges of polyatomic ions. Assign formal charge to each atom in the lewis structure. A) ph 3 b) cs 2 c) sih 4 d) pcl 3 sometimes there will be more than one correct way to draw a lewis structure for. What molecular geometry would you assign to the nitrate ion? It also has one negative charge. Web there are three lewis structures for the nitrate polyatomic ion. Draw the lewis structure for the polyatomic nitrate (no3−)anion. Names, formula, and charges of polyatomic ions. Be sure to include all resonance structures that satisfy the octet rule. Nitrogen (n) has 5 valence electrons, oxygen (o) has 6 valence electrons, and there are 3 oxygens. Nitrogen and oxygen belong to periods 5a and 6a groups respectively in the periodic table. In this video, we will go through how to draw lewis structures for polyatomic ion in five easy steps. Do you think each resonance structure contributes equally to the nitrate ion structure? Web use the idea of valence to construct lewis structures of the following compounds: A lewis structure is a way to show how atoms share electrons when they form a molecule. This problem has been solved! Determine the total number of valence electrons: Web there are three lewis structures for the nitrate polyatomic ion. What molecular geometry would you assign to the nitrate ion? Web draw the lewis structures for three resonance forms of the nitrate ion, no3 −. 88% (17 ratings) share share. Finally, draw two additional resonance structures; For the positive polyatomic ammonium, we look at the three bonding sites for nitrogen and the four possible bonding sites on the four hydrogen atoms. Lewis structures show all of the valence electrons in an atom or molecule.
SOLVED Draw the Lewis structure for the polyatomic nitrate anion. Be
How do you draw lewis structures for polyatomic ions? Socratic
[Solved] Consider the polyatomic ion nitrate, NO3 . Draw the Lewis
CH 8 CHEMISTRY LEWIS STRUCTURES POLYATOMIC IONS YouTube
[Solved] Draw Lewis structure(s) for the nitrate ion ( NO 3 ). If
Solved Draw the Lewis structure for the polyatomic nitrate

Nitrate Ion Lewis Structure How to Draw the Lewis Structure for
Solved Draw the Lewis structure for the polyatomic nitrate
[Solved] Draw all valid Lewis structures for the nitrate ion, showing

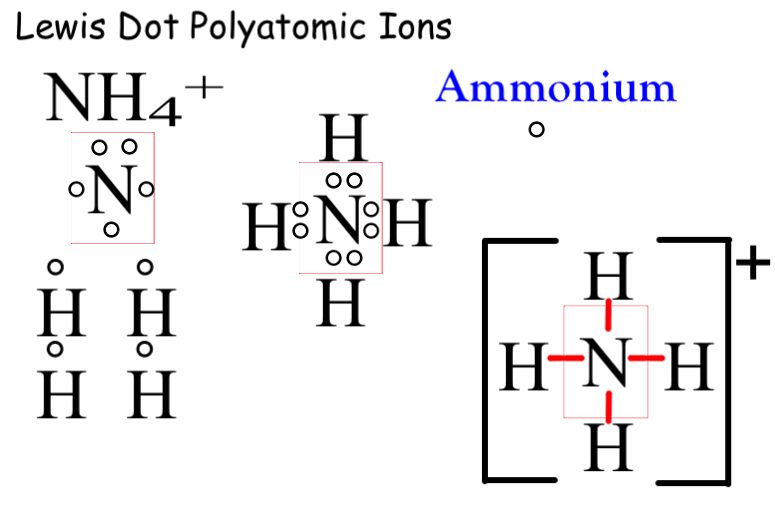
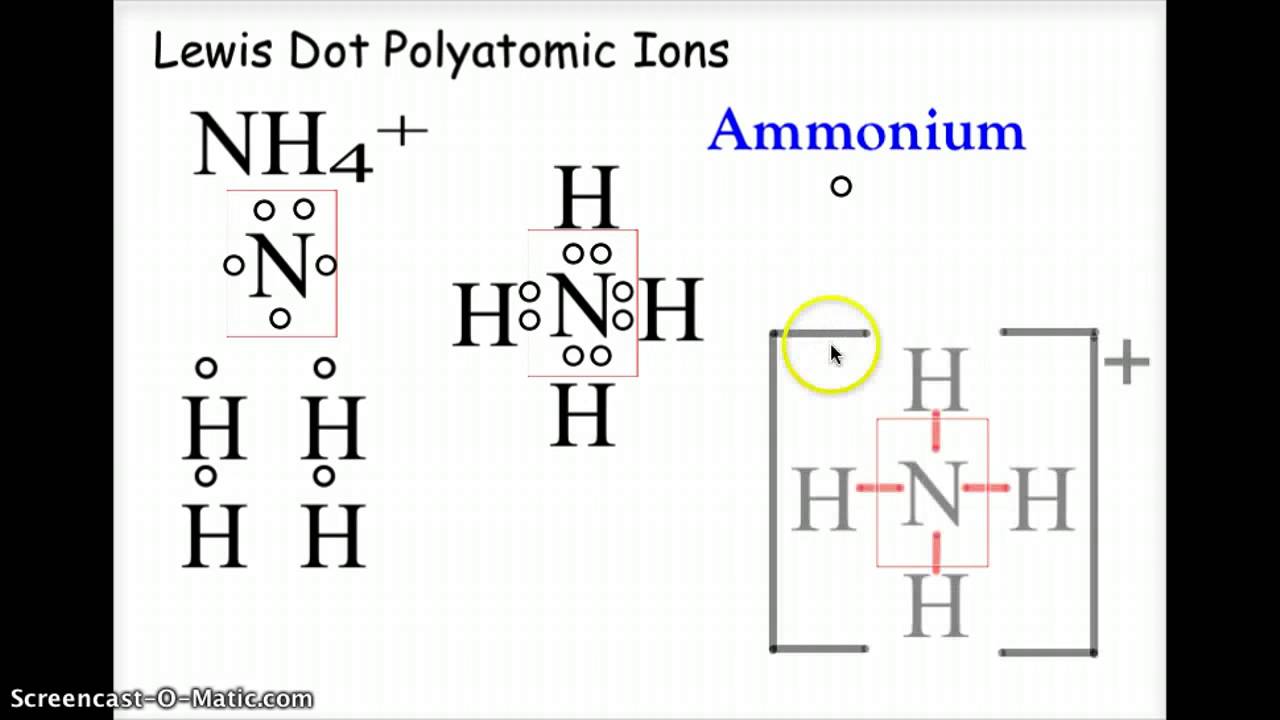
What are Polyatomic Ions? Give Examples Teachoo Concepts
This Problem Has Been Solved!
, Sal Draws The Resonance Hybrid Lewis Structure For The Nitrate Anion.
Include Electron Lone Pairs And Any Formal Charges.
In The Ion No3, There Is 1 Atom Of Nitrogen And 3 Atoms Of Oxygen.
Related Post: