Draw The Lewis Structure For The Pcl4 Ion
Draw The Lewis Structure For The Pcl4 Ion - Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary Draw lewis structures for covalent compounds. Web improve student outcomes for free! Determine the total number of valence electrons in the molecule or ion. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Comments (0) draw the lewis dot structure for the following ion:pcl4+. Web to draw any lewis structure, there is a need to follow some rules or steps which are given below. A) propanol, ch 3 ch 2 ch 2 oh b) acetic acid (or ethanoic acid), ch 3 co 2 h. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. This problem has been solved! Web draw lewis or kekule structures for the following compounds: Determine the total number of valence electrons in the molecule or ion. 5 + 4 (7) = 33. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary Web to draw any lewis structure, there is a need to follow some rules or steps which are given below. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a. Determine the total number of valence electrons in the molecule or ion. Send feedback | visit wolfram|alpha. Later, make bonding within all p and cl atoms with single covalent bonds. This widget gets the lewis structure of chemical compounds. 5 + 4 (7) = 33. A) propanol, ch 3 ch 2 ch 2 oh b) acetic acid (or ethanoic acid), ch 3 co 2 h. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web whats the lewis dot structure for pcl4+ ion. Draw the lewis structure for the pcl+4 ion. 5 + 4 (7) = 33. Draw lewis structures for covalent compounds. Here are the steps and final structure that meets the. Ion ( phosphorus tetrachloride cation), we. The valence of electrons and bonding of atoms: A) propanol, ch 3 ch 2 ch 2 oh b) acetic acid (or ethanoic acid), ch 3 co 2 h. Draw the lewis structure for the pcl+4 ion. Ion ( phosphorus tetrachloride cation), we. By the end of this section, you will be able to: Web draw a lewis structure for the following polyatomic ions: Pcl4+ is a chemical formula for tetrachloro phosphanium ion. In pcl4, the central atom is phosphorus (p). Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. This problem has been solved! 1.9k views 3 years ago. And to help you understand the lewis structure. The number of dots equals. Web to properly draw the lewis structure of pcl 4 +, follow these steps: Draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure for the pcl+4 ion. Draw lewis structures for covalent compounds. The valence of electrons and bonding of atoms: Added jun 9, 2014 by webtester in chemistry. Send feedback | visit wolfram|alpha. 1.2k views 1 year ago lewis structure. By the end of this section, you will be able to: Draw lewis structures depicting the bonding in simple molecules. Web to properly draw the lewis structure of pcl 4 +, follow these steps: C) methylamine, ch 3 nh 2 d) butanal, ch 3 ch 2 ch 2 cho. Find more chemistry widgets in wolfram|alpha. Phosphorus (p) has 5 valence electrons, and each chlorine (cl) atom has 7 valence electrons. Draw the lewis structure for the pcl a 4 a +. Determine the total number of valence electrons in the molecule. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw lewis or kekule structures for the following compounds: Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Web draw a lewis structure for the following polyatomic ions: Comments (0) draw the lewis dot structure for the following ion:pcl4+. The valence of electrons and bonding of atoms: The lewis structure for the ( pcl a 4 a +) ion. Write lewis symbols for neutral atoms and ions. Pcl4+ is a chemical formula for tetrachloro phosphanium ion. Four pairs will be used in the chemical bonds between the p and f. Here are the steps and final structure that meets the. Evaluate the total valence electrons of the pcl4+ lewis structure by adding the valence electrons on p and cl atoms. A) propanol, ch 3 ch 2 ch 2 oh b) acetic acid (or ethanoic acid), ch 3 co 2 h. View the full answer step 2.
How To Draw Lewis Structures A Step By Step Tutorial
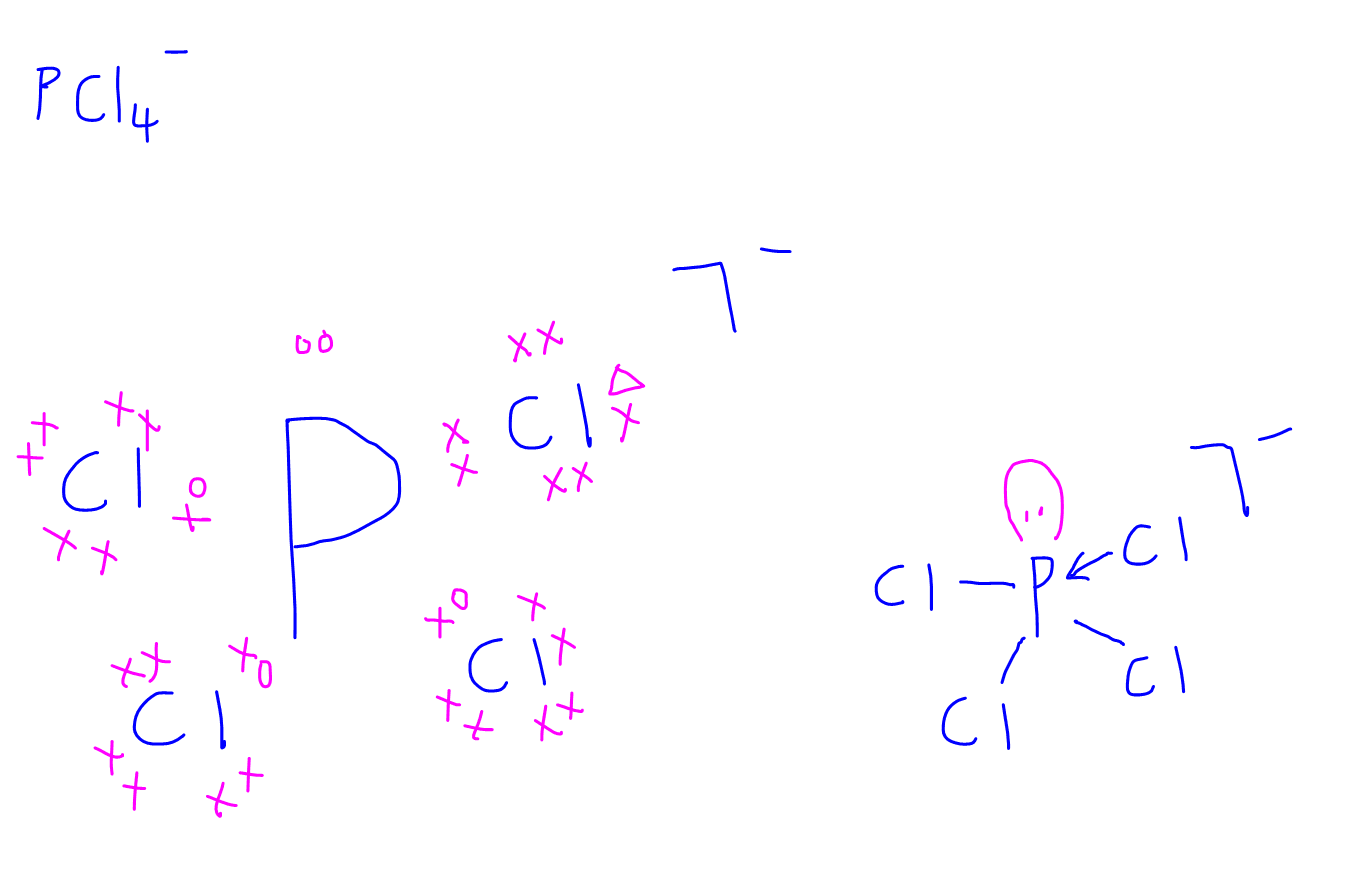
drawingdotandcrossdiagrams

What is the Lewis structure of \ce{PCl4+}? Quizlet

draw the lewis structure for the pcl 4 ion vansdisneyxvansoldskool

Lewis Dot Structure For Pcl4+ Draw Easy

How to Draw the Lewis Structure for PCl4+ YouTube
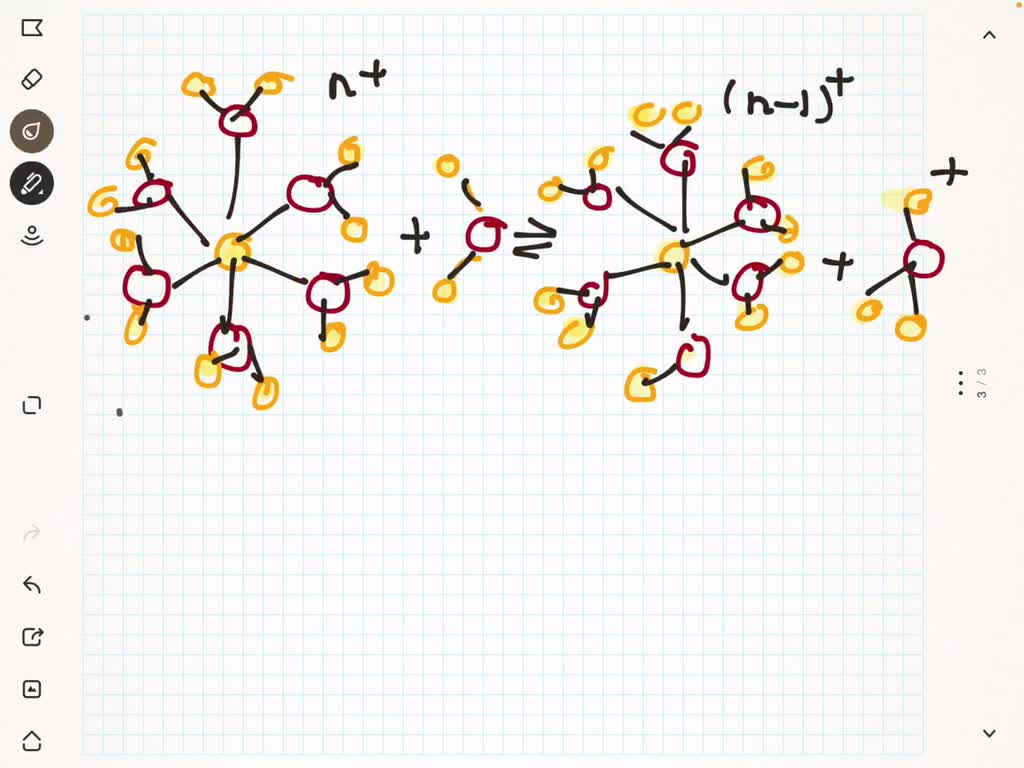
SOLVED(a) The following diagram represents the reaction of PCl4

Draw the Lewis Structure for the Pcl+4 Ion
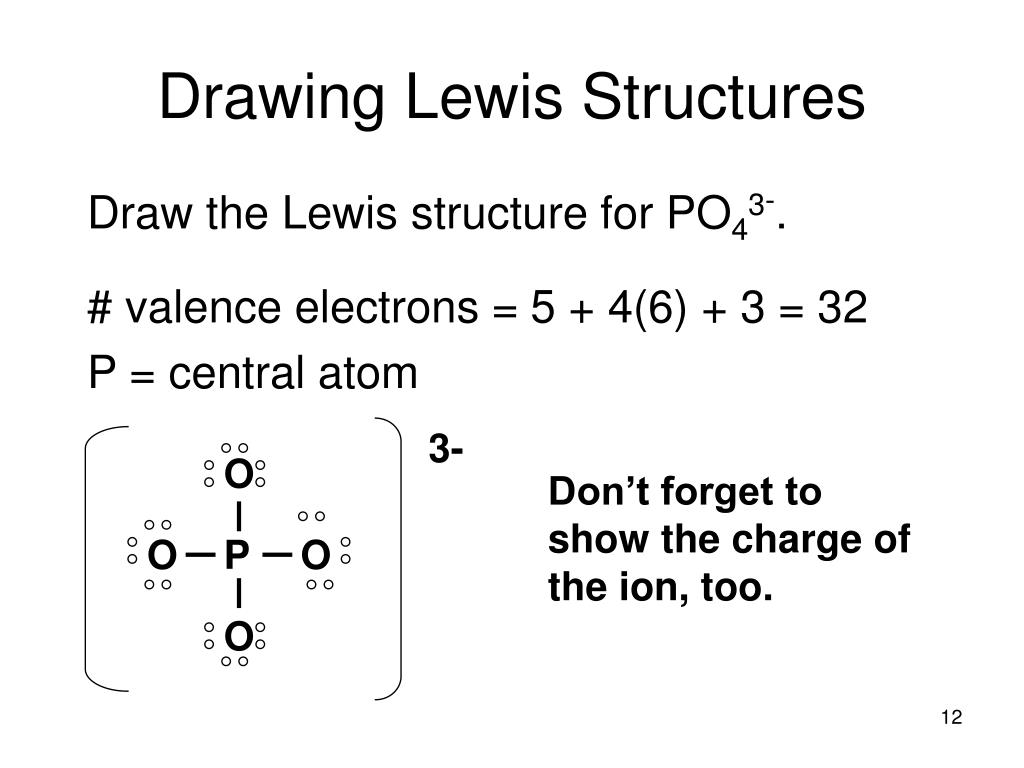
Ppt Drawing Lewis Structures A Tutorial On Writing Lewis Dot 193

draw the lewis structure for the pcl 4 ion vansdisneyxvansoldskool
5 + 4 (7) = 33.
Therefore, The Total Number Of Valence Electrons In Pcl4 Is:
This Widget Gets The Lewis Structure Of Chemical Compounds.
A Lewis Structure Is A Way To Show How Atoms Share Electrons When They Form A Molecule.
Related Post: