Draw The Lewis Structure For The Nf3
Draw The Lewis Structure For The Nf3 - Nf 3 (nitrogen trifluoride) is very similar to the ncl 3 and nh 3 lewis structure. The nitrogen atom (n) is at the center and it is surrounded by 3 fluorine atoms (f). It consists of one nitrogen and three fluorine atoms. Once the lewis structure is drawn, the shape of the nf 3. Begin by identifying the number of valence electrons for each atom in the nf3 molecule. Web nf3 lewis structure. (valence electrons are the electrons that are present in the outermost orbit of any. Nitrogen has 5 valence electrons, and each fluorine atom has 7 valence electrons, giving us a total of 26 valence electrons for nf3. Now, let’s take a closer look at each step mentioned above. 151k views 10 years ago. Nf 3 (nitrogen trifluoride) is very similar to the ncl 3 and nh 3 lewis structure. N ( z= 7) = [he] 2s 2 2p 3 ie 5 valence electrons are present. Web here’s how you can easily draw the nf 3 lewis structure step by step: Draw the lewis structure for nf3 (nitrogen is the central atom) and select. Web drawing the lewis structure of nf 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial. Once the lewis structure is drawn, the shape of the nf 3. Draw the lewis structures for nf3 and if3. In the lewis structure for nf 3 there are a total. Web a video explanation of how to draw the lewis dot structure for nitrogen trifluoride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond. F (z = 9) = [he] 2s 2 2p 5 ie only 7 electrons are. This widget gets the lewis structure of chemical compounds. First count the number of valence. Nitrogen trifluoride is a chemical molecule having one atom of nitrogen and three atoms of fluorine. To create the lewis structure of nf3, follow these steps: There are 3 steps to solve this one. This problem has been solved! Web drawing the lewis structure of nf3. Web nf3 lewis structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. After drawing the lewis structure of nf 3, you can decide shape of the nf 3 molecule. #1 draw a rough skeleton structure. Lewis structures show all of the valence electrons in an atom or molecule. E) draw the dipole moments on the lewis structure. Draw the lewis structures for nf3 and if3. #1 draw a rough skeleton structure. Added jun 9, 2014 by webtester in chemistry. Begin by identifying the number of valence electrons for each atom in the nf3 molecule. Now, let’s take a closer look at each step mentioned above. Web steps of drawing nf3 lewis structure. This widget gets the lewis structure of chemical compounds. In lewis structure, we try to find a suitable pictorial representation of a molecule to have an idea of the chemical bonding occurring inside the molecular structure. Added jun 9, 2014 by webtester. Web nf3 lewis structure. Web lewis structure of nf3 contains three single bonds between the nitrogen (n) atom and each fluorine (f) atom. Let’s draw the nf 3 lewis structure step by step; Since in the outermost shell of the nitrogen 5 electrons are present. Web the lewis structure is a diagram that illustrates the bonding between atoms and the. Once the lewis structure is drawn, the shape of the nf 3. In order to find the total valence electrons in nf3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as fluorine atom. Web lewis structure of nf 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms. Each step of drawing the lewis structure of nf 3 is explained in detail in this tutorial. Nitrogen has 5 valence electrons, and each fluorine atom has 7 valence electrons, giving us a total of 26 valence electrons for nf3. Draw the lewis structures for nf3 and if3. #3 if needed, mention formal charges on the atoms. Nitrogen trifluoride is. N ( z= 7) = [he] 2s 2 2p 3 ie 5 valence electrons are present. In the nf 3 lewis structure (and all structures) hydrogen goes on the outside. D) what are the bond angles? In the case of nf3, the lewis structure comprises a nitrogen atom at the center and three fluorine atoms surrounding it, each bonded to the nitrogen atom through a single bond. Web steps of drawing nf3 lewis structure. In order to find the total valence electrons in nf3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as fluorine atom. The valence electrons are the electrons in the. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. E) draw the dipole moments on the lewis structure. #2 mention lone pairs on the atoms. Web a video explanation of how to draw the lewis dot structure for nitrogen trifluoride, along with information about the compound including formal charges, polarity, hybrid orbitals, shape, and bond. Web nf3 lewis structure. Fluorine is a group viia element and has seven electrons in its last shell (valence shell). A) draw the correct lewis structure for nitrogen trifluoride, nf3. Each step of drawing the lewis structure of nf 3 is explained in detail in this tutorial. Web drawing the lewis structure of nf 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial.
lewis structure for nitrogen trifluoride

draw lewis structures for the following molecules nf3 csnplacement
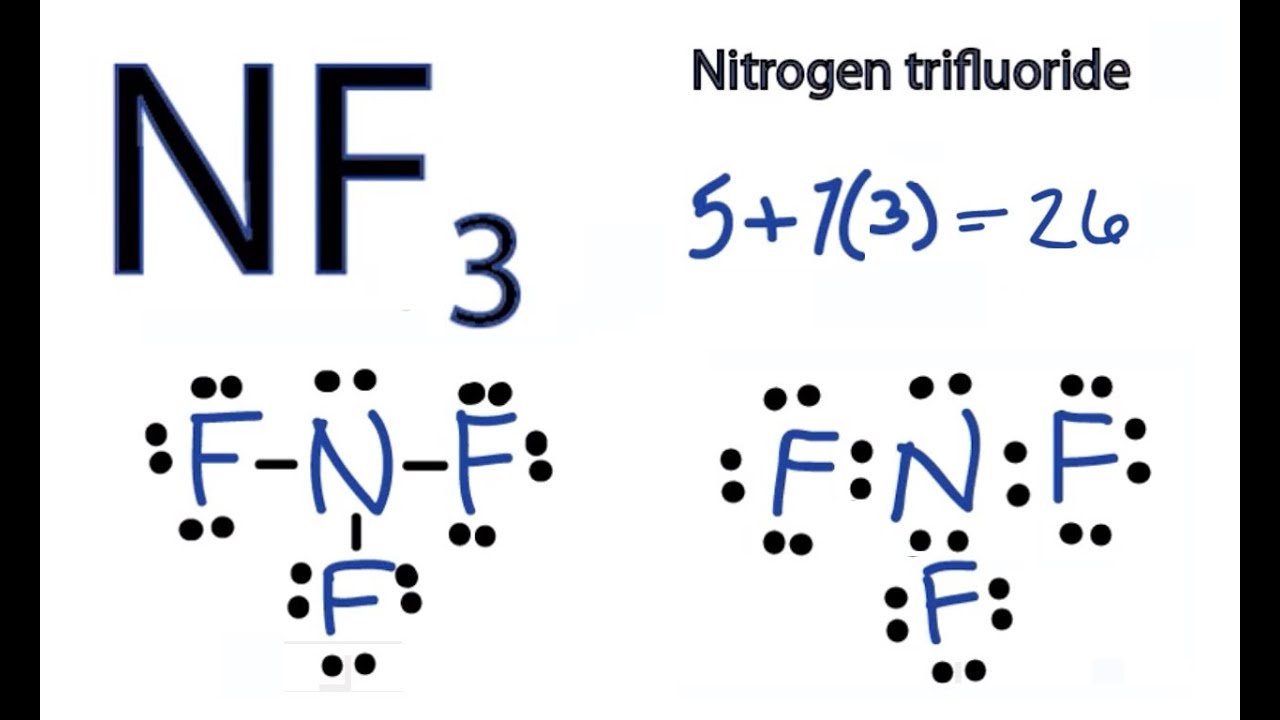
NF3 Lewis Structure How to Draw the Dot Structure for NF3 (Nitrogen

How to draw NF3 Lewis Structure? 4

Draw The Lewis Structure Of The Molecule Nf3 Fotodtp

NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and

NF3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
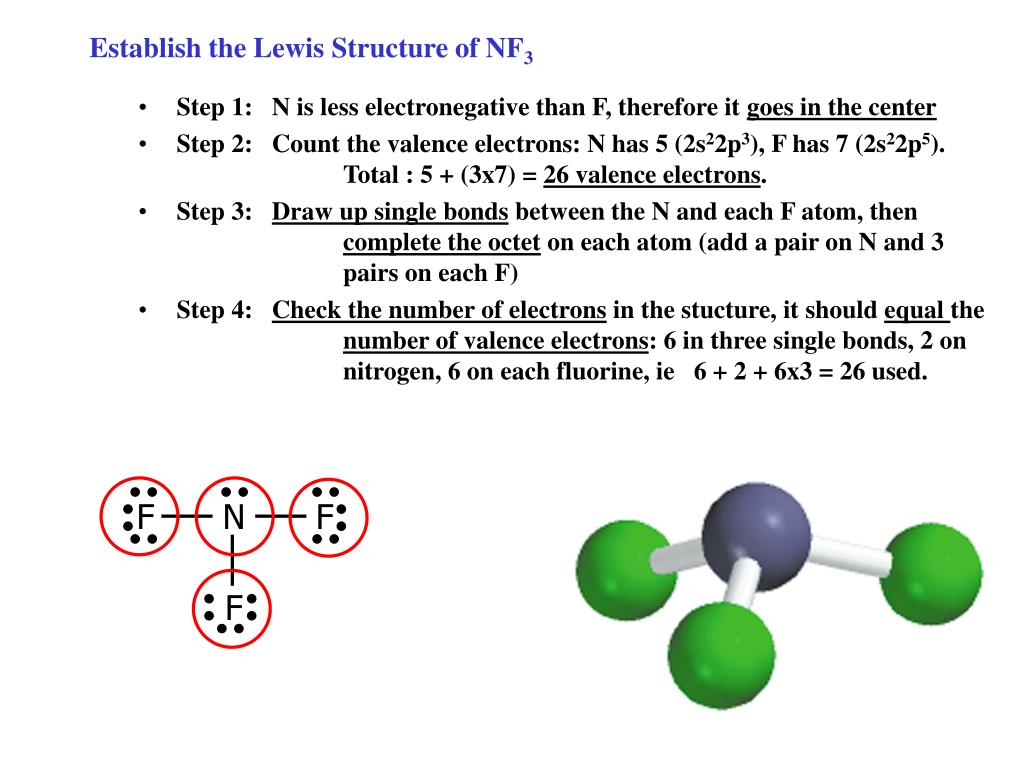
PPT Establish the Lewis Structure of NF 3 PowerPoint Presentation

How to draw NF3 Lewis Structure? 3
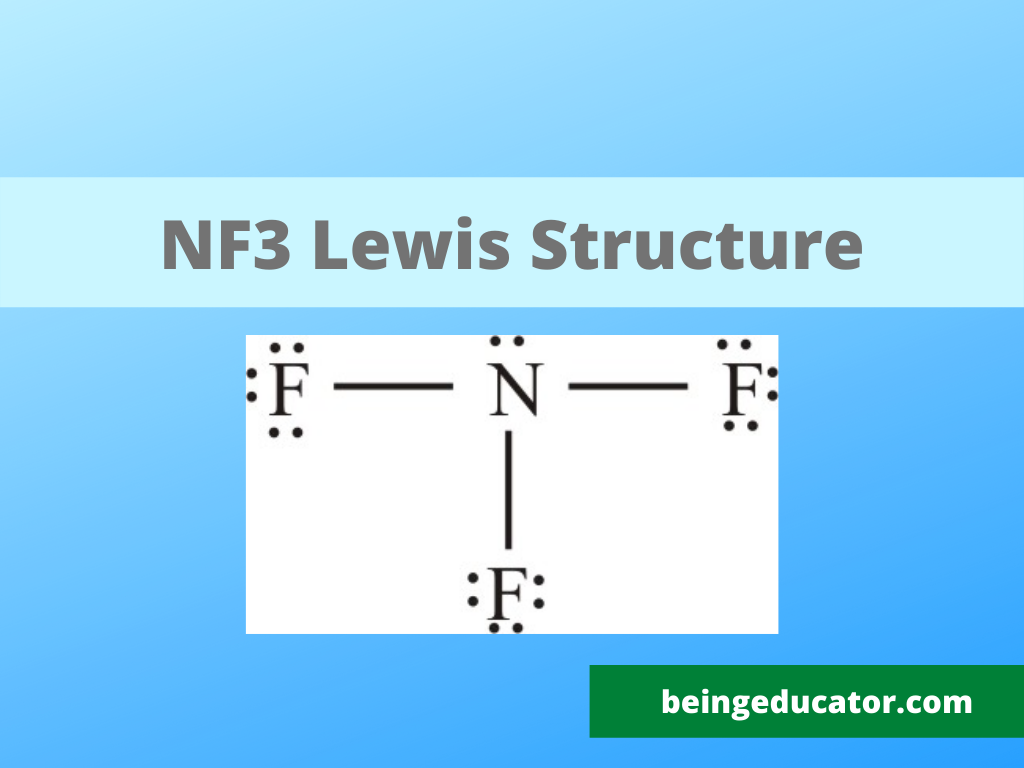
NF3 Lewis Structure
There Are 3 Steps To Solve This One.
Web Lewis Structure Of Nf 3 Can Be Drawn By Starting From Valence Electrons Of Nitrogen And Fluorine Atoms In Several Steps.
First Count The Number Of Valence Electrons In The Molecule Nf 3.
B) What Is The Electron Group Arrangement?
Related Post: