Draw The Lewis Structure For The Iodine Difluoride Ion
Draw The Lewis Structure For The Iodine Difluoride Ion - (note, all the atoms are bonded to the central i atom.) steric number octet rule exceptions choose at least one answer. Now, let’s take a closer look at each step. The iodine atom as well as both the fluorine. Web page contents show. Count the total number of valence electrons: Iodine (i) has 7 valence electrons, and each fluorine (f) atom has 7 valence electrons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. This problem has been solved! #3 if needed, mention formal charges on the atoms. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. This problem has been solved! The iodine atom (i) is at the center and it is surrounded by 2 fluorine atoms (f). Web lewis diagram of xenon difluoride (worked example) (video) | khan academy. So, if you are ready to go with these 6 simple steps, then let’s dive. In some molecules, the central atom exceeds the octet rule (is. #3 if needed, mention formal charges on the atoms. Web lewis diagram of xenon difluoride (worked example) (video) | khan academy. The iodine atom as well as both the fluorine. Count the total number of valence electrons: The electronic configuration of kr four d 10, five s two, and b five is why iodine has it. 59k views 10 years ago. Which statements are correct regarding its lewis structure? Iodide is the name of the person. Draw the lewis structure for the iodine difluoride ( if, ion. In this case, the central atom is iodine. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Now, let’s take a closer look at. #1 draw a rough skeleton structure. Where, v= no of valance electrons, b= no of bonding electrons, n= no of nonbonding electrons. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The structure of hydrogen has to be drawn. #2 mention lone pairs on the atoms. The central atom in lewis structures is usually the least electronegative one. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web draw the lewis structure for the iodine difluoride (if,) ion. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation. Web may 23, 2023 by jay rana. Since it's an ion, we need to add one more electron. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web page contents show. (note, all the atoms are bonded to the central i atom.) steric number octet rule exceptions choose at least one answer. Unveiling the versatile metal's role in modern technology. Hydrogen has an elected configuration. In this case, the central atom is iodine. The number of dots equals the number of valence. Now, let’s take a closer look at each step. Hydrogen has an elected configuration. 59k views 10 years ago. Web lewis diagram of xenon difluoride (worked example) (video) | khan academy. The structure of hydrogen has to be drawn. In some molecules, the central atom exceeds the octet rule (is surrounded by more than. Web lewis diagram of xenon difluoride (worked example) (video) | khan academy. Iodide is the name of the person. Web draw the lewis structure for the iodine difluoride (if,) ion. Count the total number of valence electrons: Web may 23, 2023 by jay rana. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Iodide is the name of the person. Where, v= no of valance electrons, b= no of bonding electrons, n= no of nonbonding electrons. The electronic configuration of kr four d 10, five s two, and b five is why iodine has it. So, if you are ready to go with these 6 simple steps, then let’s dive. The number of valence electrons in i f x 2 x −. Hydrogen has an elected configuration. Web lewis diagram of xenon difluoride (worked example) (video) | khan academy. Draw the lewis structure for the iodine difluoride ( if, ion. Web may 23, 2023 by jay rana. In some molecules, the central atom exceeds the octet rule (is surrounded by more than. #1 draw a rough skeleton structure. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Which statements are correct regarding its lewis structure? The central atom in lewis structures is usually the least electronegative one. (note, all the atoms are bonded to the central i atom.) steric number octet rule exceptions choose at least one answer.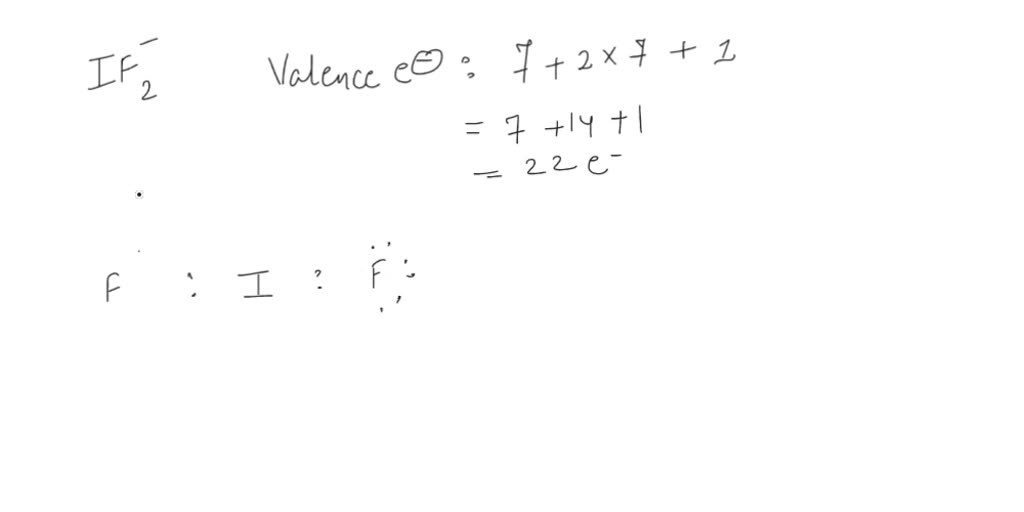
SOLVED Draw the Lewis structure for the iodine difluoride ([Fz) ion.

Lewis Structure For Iodine
Iodine Lewis Dot Diagram
Draw the Lewis structure for the iodine difluoride IF… SolvedLib

Lewis Structure For Iodine
(Get Answer) Draw The Lewis Structure For The Iodine Difluoride (IF 7

Lewis Dot Diagram Iodine
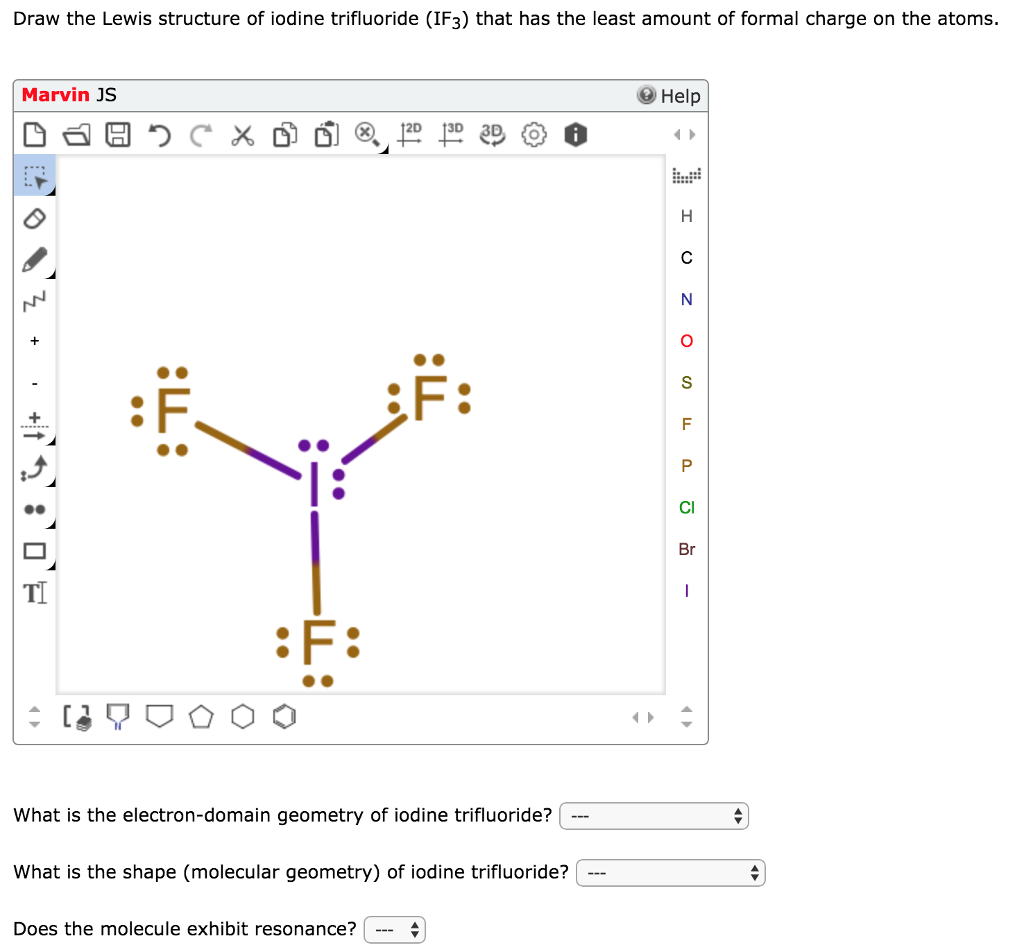
Lewis Structure For Iodine
Solved Draw the Lewis structure for the iodine difluoride
Solved Draw the Lewis structure for the iodine difluoride
The Astute Reader May Have Noticed Something:
Iodine (I) Has 7 Valence Electrons, And Each Fluorine (F) Atom Has 7 Valence Electrons.
Shared Pairs Of Electrons Are Drawn As Lines Between Atoms, While Lone Pairs Of Electrons Are Drawn As.
Since It's An Ion, We Need To Add One More Electron.
Related Post: