Draw The Lewis Structure For Sih4
Draw The Lewis Structure For Sih4 - You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure for co. See solution check out a sample q&a here. Sih4 lewis structure lone pairs; Write lewis symbols for neutral atoms and ions. In order to find the total valence electrons in sih4 molecule, first of all you should know the valence electrons present in silicon atom as. Remember that hydrogen (h) only needs two valence electrons. By the end of this section, you will be able to: С chemdoodle b is sih4 polar or nonpolar? 8 + (2 × 7) = 22 xef 6: Sih4 lewis structure lone pairs; Start with the silicon atom in the center and draw four single bonds to the hydrogen atoms. The carbon—carbon bonds are labeled 1, 2, and 3. By the end of this section, you will be able to: Draw the lewis structure for sih4. Draw the lewis structure of sih4. Include lone pairs, as needed. Web drawing the lewis structure for sih 4. A use the references to access important values if needed for this question. For the sih4 structure use. Draw the lewis structure for co. Draw the lewis structure of sih4. Draw a lewis structure for i. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of sih4 contains four single bonds between each silicon (si) and hydrogen (h) atoms. Sih4 lewis structure octet rule; Web here, i have explained 6 simple steps to draw the lewis dot structure of sih4 (along with images). Include all lone pairs of electrons. Web drawing the lewis structure for sih 4. Web to draw the lewis structure for sih4, follow these steps: Draw a lewis structure for i. Draw a lewis structure for silane (sih4) and predict its molecular geometry. Web by using the following steps, you can easily draw the lewis structure of sih 4: Solved in 2 steps with 1 images. How to draw lewis structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. P aste ору [ 1+ ? Web sih4 lewis structure. Draw a lewis structure for silane (sih4) and predict its molecular geometry. (a) how many hydrogen atoms are in the molecule? Draw the lewis structure of sih4. Web to properly draw the sih 4 lewis structure, follow these steps: 22k views 10 years ago. Draw the lewis structure for co. Web drawing the lewis structure for sih 4. How to draw lewis structure for sih4; You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Br ch₂12 cio nh4 sih4 h₂o* give detailed solution with structures, don't…. Find the total valence electrons in sih4 molecule. Welcome back to our channel, and in today’s video, we will help you do sih4 lewis structure. Web sih4 lewis structure. The lewis structure for sih 4 has 8 valence electrons available to work with. Include all lone pairs of electrons. Web we have got our most suitable lewis structure sketch for sih4. Draw the lewis structure of sih4. Start with the silicon atom in the center and draw four single bonds to the hydrogen atoms. Web by using the following steps, you can easily draw the lewis structure of sih 4: Remember that hydrogen (h) only needs two valence electrons. P aste opy с 7 с chemdoodle b is xeo3 polar or nonpolar? Select draw rings more iii. P aste opy с 7 с chemdoodle b is xeo3 polar or nonpolar? A lewis structure is a way to show how atoms share electrons when they form a molecule. Sih 4 is similar to sif 4. The carbon—carbon bonds are labeled 1, 2, and 3. Each hydrogen atom should have two electrons around it, one from the bond and one as a lone pair. Si has 4 valence electrons and each h has 1 valence electron, so the total is 4 + 4 (1) = 8 valence electrons. Web to draw the lewis structure for sih4, follow these steps: Calculate the number of valence electrons: Remember that hydrogen (h) only needs two valence electrons. Draw the lewis structure of sih4. Sih4 lewis structure octet rule; Lewis structures show all of the valence electrons in an atom or molecule. Web by using the following steps, you can easily draw the lewis structure of sih 4: #3 calculate formal charge and check stability (if there are no lone pairs and octet is already completed on central atom) let’s one by one discuss each step in detail. С chemdoodle b is sih4 polar or nonpolar? Draw lewis structures depicting the bonding in simple molecules.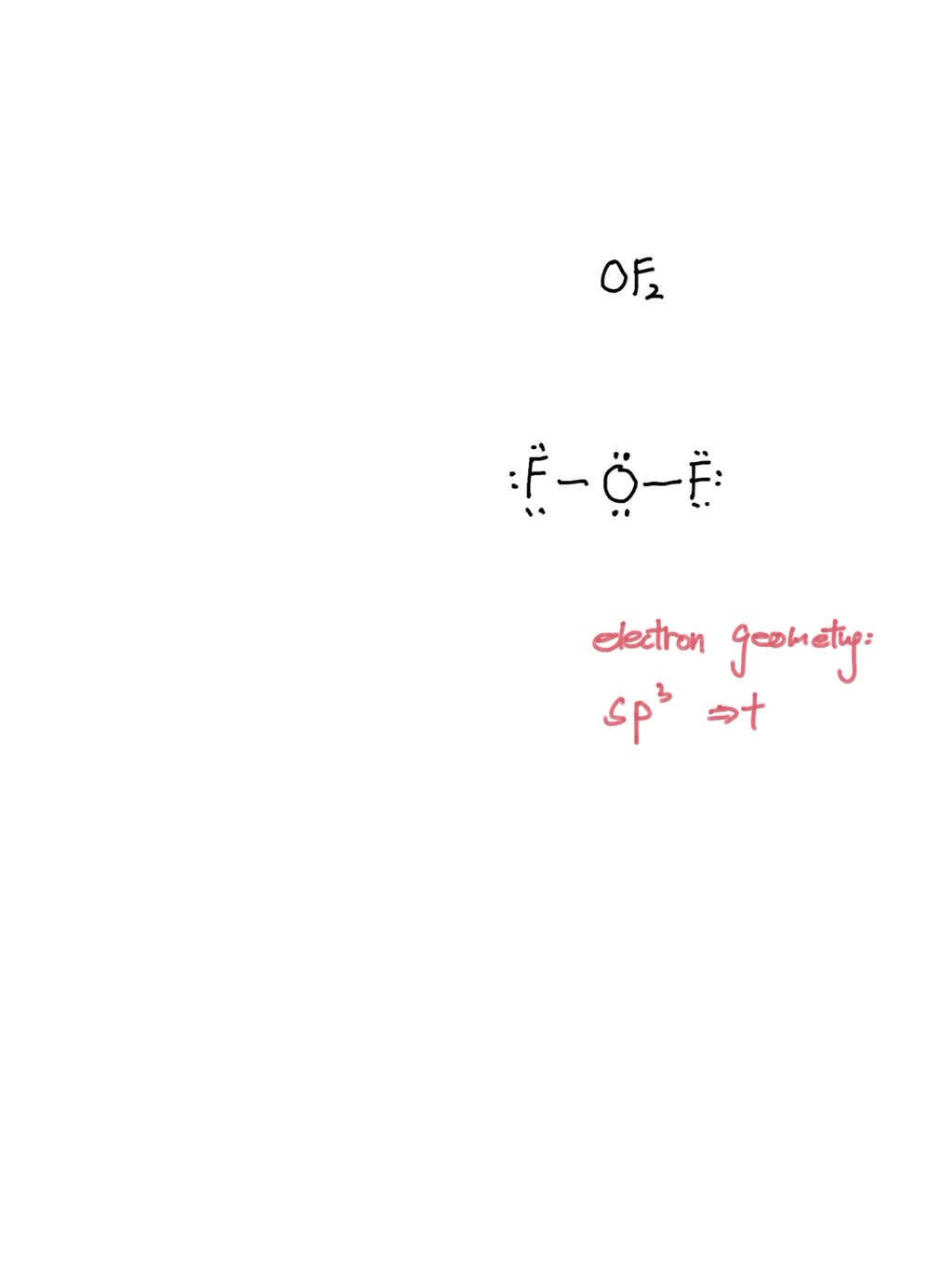
SOLVED Draw a Lewis structure for silane (SiH4) and predict its

SiH4 Lewis Structure (Silicon Tetrahydride) YouTube

Is SiH4 Polar or Nonpolar? Techiescientist

diagrama de lewis del enlace siH4 Brainly.lat
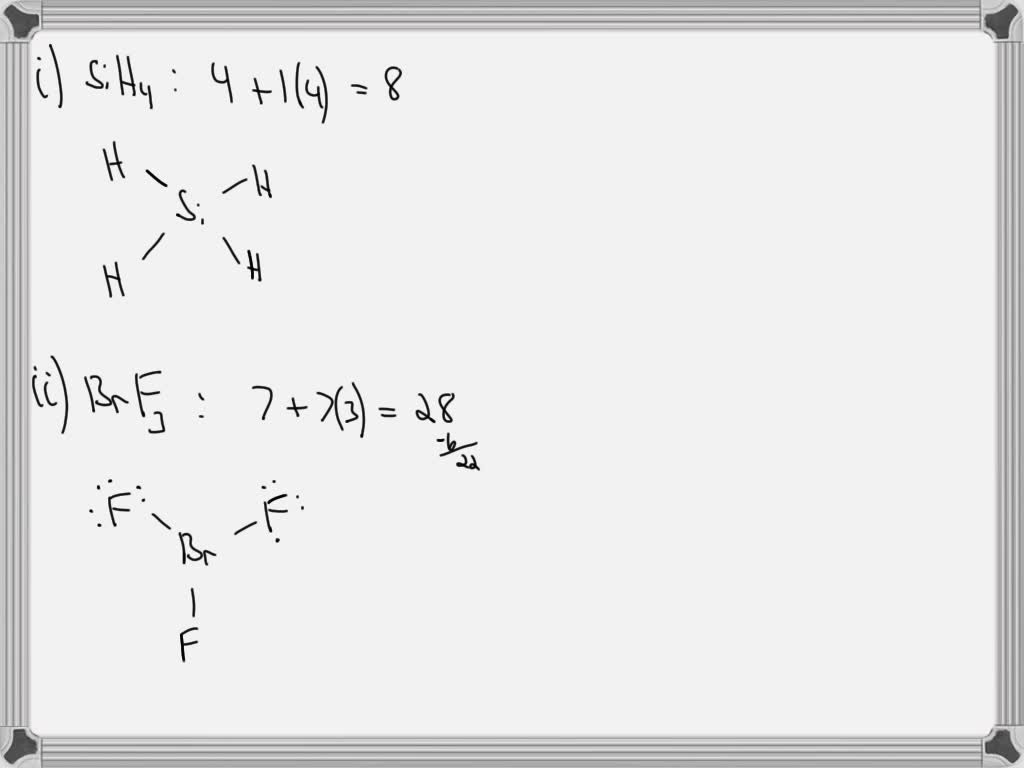
SOLVED Draw a Lewis structure for the following three molecules
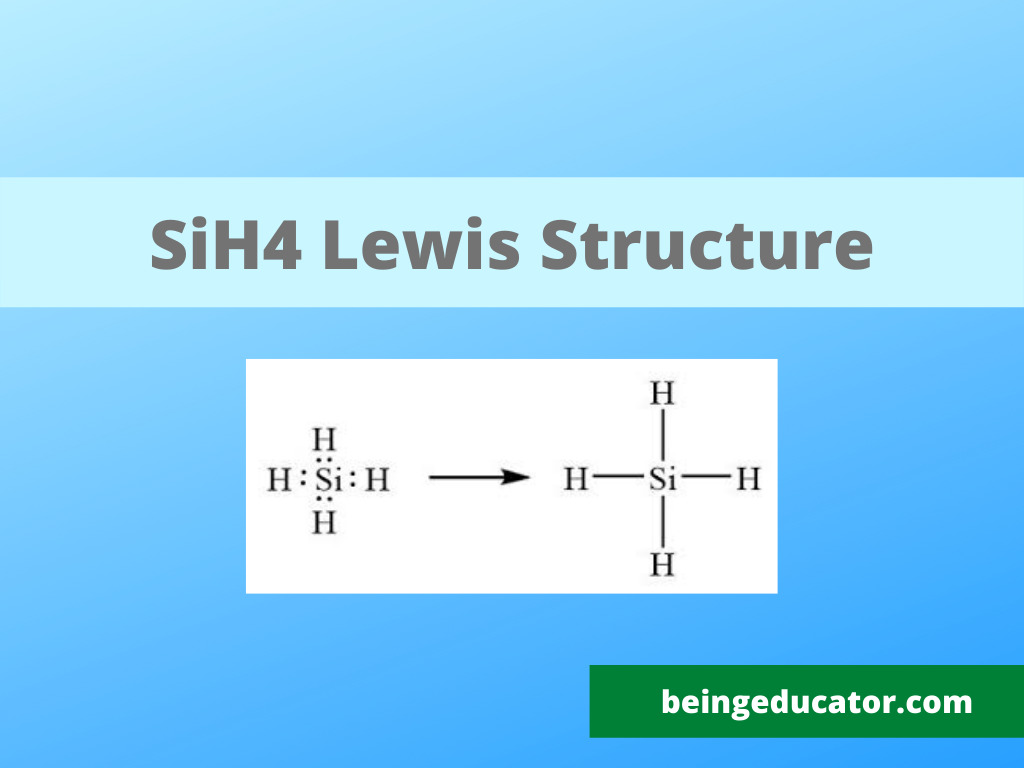
Lewis Structure, Hybridization, Polarity and Molecular Geometry of SiH4
[Solved] Draw the Lewis structure for SiH 4 in the window below and

SiH4 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
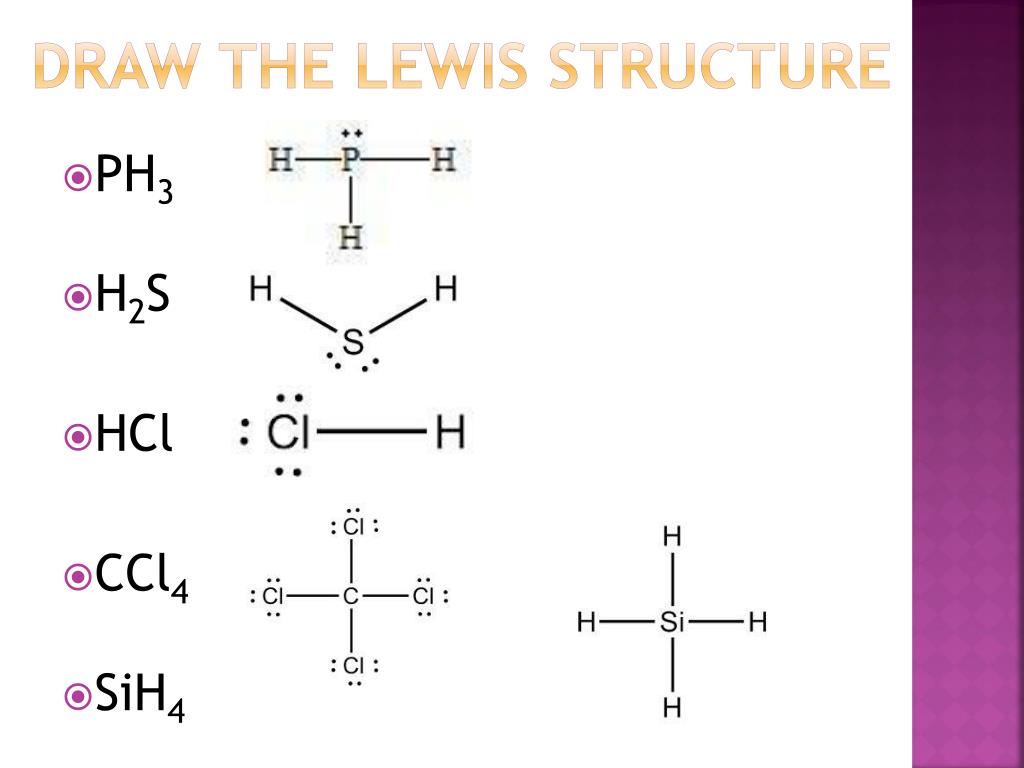
PPT Lewis Structures PowerPoint Presentation, free download ID6114495

Draw The Lewis Structure For Sih4 What Is Its Molecular Geometry Fotodtp
Let Us See The Following Topics In This Article.
Web A Draw The Lewis Structure For Sih4 In The Window Below And Then Decide If The Molecule Is Polar Or Nonpolar.
22K Views 10 Years Ago.
Follow This Video To Know The Detailed Method An.
Related Post: