Draw The Lewis Structure For Ch3Cl
Draw The Lewis Structure For Ch3Cl - The atomic number of hydrogen (h) is 1, so its electronic configuration is 1s1. Web in the lewis structure of ch 3 cl, carbon is at the central position and all the other atoms around it. Select the intermolecular forces present between ch3cl molecules. Through lewis structure, we can predict that bonds formed between atoms are single, double, or triple bonds. Web check me out: The geometry around the central angle is ______. Web the essay is divided into three parts: In order to draw the lewis structure of ch3cl, first of all you have to find the total number of valence electrons present in the ch3cl molecule. Web hey guys!in this video, we are going to learn about the lewis dot structure of chloromethane or methyl chloride having a chemical formula of ch3cl. To begin with the lewis structure of ch3cl, first, we need to determine the electronic configuration of each participating atom. Web how to draw lewis structure of ch3cl? Web by using the following steps, you can easily draw the lewis structure of ch 3 cl: On the periodic table, carbon group 4 or 14, 4 valence electrons. #3 calculate and mark formal charges on the atoms, if required. 102k views 10 years ago. The bond angles of carbon with hydrogen and chlorine atoms are 109.5 degrees. The central atom is ______. Web this widget gets the lewis structure of chemical compounds. Let’s discuss each step in more detail. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web drawing lewis structures for molecules with one central atom: There are a total of 4 electron density regions around the central c atom in the ch3cl lewis structure. Let’s discuss each step in more detail. To begin with the lewis. #1 first draw a rough sketch. 102k views 10 years ago. In order to draw the lewis structure of ch3cl, first of all you have to find the total number of valence electrons present in the ch3cl molecule. Use these steps to correctly draw the ch 3 cl lewis structure: Draw the lewis structure for ch3cn. Web lewis structure is drawn by dots which represent the valence electrons assigned around the elements present in the molecules. The central atom is ______. The bond angles of carbon with hydrogen and chlorine atoms are 109.5 degrees. Here, the given molecule is ch3cl. Let’s discuss each step in more detail. The geometry around the central atom is ______. Through lewis structure, we can predict that bonds formed between atoms are single, double, or triple bonds. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web the essay is divided into three parts: Web in the lewis structure of ch 3 cl, carbon is at. Number of steps can be changed according the complexity of the molecule or ion. Use these steps to correctly draw the ch 3 cl lewis structure: Hydrogens always go on the outside. To begin with the lewis structure of ch3cl, first, we need to determine the electronic configuration of each participating atom. #4 calculate formal charge and check stability (if. Drawing neutral atoms, drawing positively charged atoms, and drawing negatively charged atoms. Carbon will go in the center. Web how to draw lewis structure for ch3cl chloromethane (methylchloride)lewis structure: Web in the lewis structure of ch 3 cl, carbon is at the central position and all the other atoms around it. Web this widget gets the lewis structure of chemical. Web the essay is divided into three parts: To use lewis dot symbols to explain the stoichiometry of a compound. Web hey guys!in this video, we are going to learn about the lewis dot structure of chloromethane or methyl chloride having a chemical formula of ch3cl. Hydrogen's in group 1 but we've got 3 hydrogens. Web steps of drawing ch3cl. The atomic number of hydrogen (h) is 1, so its electronic configuration is 1s1. #1 first draw a rough sketch. The geometry around the central atom is ______. Web steps of drawing lewis structure of ch 3 cl. Calculate the total number of valence electrons. Let’s discuss each step in more detail. Web in the lewis structure of ch 3 cl, carbon is at the central position and all the other atoms around it. There are 26 valence electrons. The geometry around the central angle is ______. There are a total of 4 electron density regions around the central c atom in the ch3cl lewis structure. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Here, the given molecule is ch3cl. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Web 6 steps to draw the lewis structure of ch3cl step #1: In order to draw the lewis structure of ch3cl, first of all you have to find the total number of valence electrons present in the ch3cl molecule. #2 mark lone pairs on the atoms. To use lewis dot symbols to explain the stoichiometry of a compound. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web hey guys!in this video, we are going to learn about the lewis dot structure of chloromethane or methyl chloride having a chemical formula of ch3cl. On the periodic table, carbon group 4 or 14, 4 valence electrons. The bond angles of carbon with hydrogen and chlorine atoms are 109.5 degrees.
Lewis Structure Ch3cl

Ch3cl lewis structure, Characteristics13 Must To Know Facts Lambda Geeks

CH3Cl Lewis Structure How to Draw the Lewis Structure for CH3Cl

How to draw CH3Cl Lewis Structure? Science Education and Tutorials
[DIAGRAM] Lewis Diagram Ch3cl
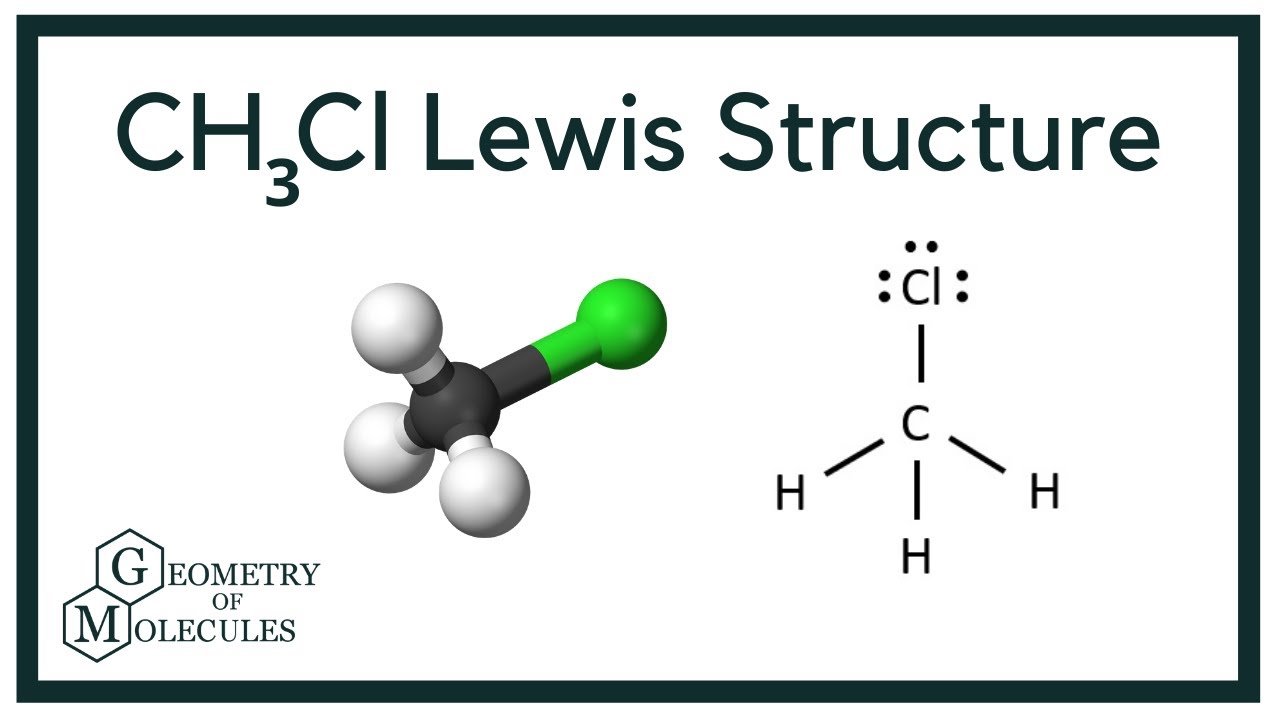
What Is Ch3cl Lewis Structure?

CHCl3 Lewis Structure, Geometry, Hybridization, and Polarity

How to draw CH3Cl Lewis Structure? Science Education and Tutorials

Lewis Structure Of Ch3cl

Lewis Structure For Ch3cl
For The Ch3Cl Structure Use The Periodic Table.
Draw The Lewis Structure For Ch3Cl.
Carbon Will Go In The Center.
To Begin With The Lewis Structure Of Ch3Cl, First, We Need To Determine The Electronic Configuration Of Each Participating Atom.
Related Post:
![[DIAGRAM] Lewis Diagram Ch3cl](http://www.sliderbase.com/images/referats/77b/(9).PNG)