Draw The Lewis Structure For A Oxygen Molecule
Draw The Lewis Structure For A Oxygen Molecule - The atomic number of oxygen is 8 and its electronic configuration is 2,6. By the end of this section, you will be able to: Note that each atom must contribute one electron to the bond. Web chemical bonding and molecular geometry. Solution for 7) draw the correct lewis structure for the following molecular species: Here, the given molecule is o2 (oxygen). Drawing the lewis structure for o2. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. 6.1 lewis symbols and structures. Note that diatomic oxygen is often called molecular oxygen or just. Draw lewis structures depicting the bonding in simple molecules. The only thing smaller than atoms is their subatomic particles; This diagram shows bonds with the help of lines and lone pairs of. Furthermore, this structure also helps with determining the lone electrons existing within the molecule and how they. Web april 5, 2022 by mansi sharma. Draw lewis structures for molecules. Not even under a complex microscopic can we view the individual electrons that surround an atom’s nuclei. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Web 22k views 3 years ago. Draw lewis structures for molecules. Atoms can form more than one bond. Web steps of drawing o2 lewis structure. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on. Br ch₂12 cio nh4 sih4 h₂o* give detailed solution with structures, don't…. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Note that each atom must contribute one electron to the bond. By the end of this section, you will be able to: Web for the lewis structure for o 2 you're going to need a double bond in order to for each oxygen atom to. Write lewis symbols for neutral atoms and ions. Furthermore, this structure also helps with determining the lone electrons existing within the molecule and how they will be acting in a bond formation. Write lewis symbols for neutral atoms and ions. There are 12 valence electrons available for the lewis structure for o 2. Note that each atom must contribute one. (note that we denote ions with brackets around the. Note that each atom must contribute one electron to the bond. There are many things to learn when we draw the o2 lewis structure. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Find the total valence electrons in o2 molecule. For the o2 structure use the periodic table to find the total number of valence. 6 steps to draw the lewis structure of o2. In the lewis structure of n2 molecule, a double bond is located between oxygen atoms and each oxygen atom has two lone pairs in their valence shells. Web for the lewis structure for o 2 you're. By the end of this section, you will be able to: Here, the given molecule is o2 (oxygen). Find the total valence electrons in o2 molecule. By the end of this section, you will be able to: Note that diatomic oxygen is often called molecular oxygen or just. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Solved in 2 steps with 1 images. For the o2 structure use the periodic table to find the total number of valence electrons for the o2. In all. The atomic number of oxygen is 8 and its electronic configuration is 2,6. 6 steps to draw the lewis structure of o2. Draw lewis structures depicting the bonding in simple molecules. In order to find the total valence electrons in o2 (oxygen) molecule, first of all you should know the valence electrons present in a single oxygen atom. This diagram. Write lewis symbols for neutral atoms and ions. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. (valence electrons are the electrons that are present in the outermost orbit of any. See solution check out a sample q&a here. 150k views 10 years ago. Web 22k views 3 years ago. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web steps of drawing o2 lewis structure. Chemical bonding and molecular geometry. By the end of this section, you will be able to: In the lewis structure of n2 molecule, a double bond is located between oxygen atoms and each oxygen atom has two lone pairs in their valence shells. Find the total valence electrons in o2 molecule. Atoms can form more than one bond. 6 steps to draw the lewis structure of o2. For the o2 structure use the periodic table to find the total number of valence.How to Draw O2 Lewis Structure? 2

O2 2 Lewis Structure How to Draw the Lewis Structure for O2 2 YouTube
Lewis Dot Structure For Oxygen Atom slidesharedocs
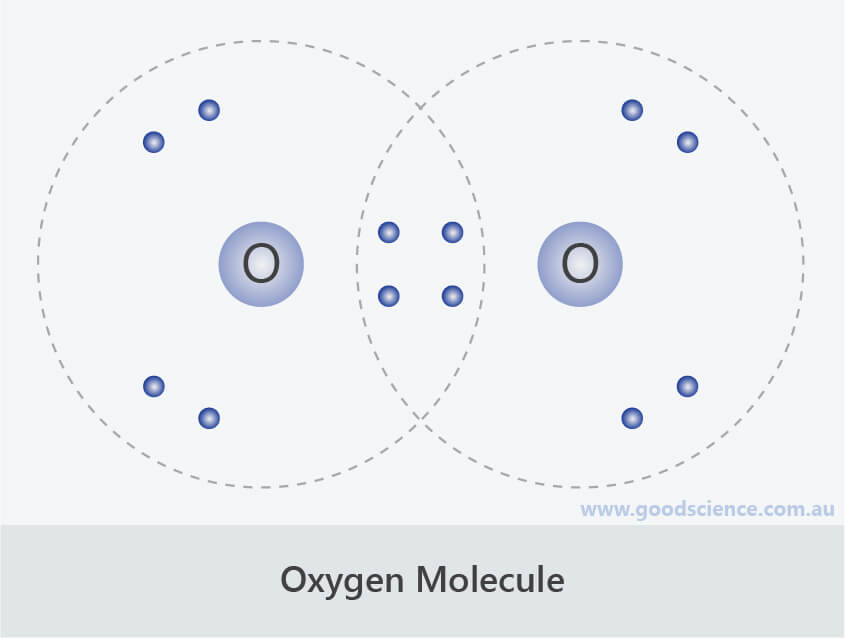
Lewis Structure Of Oxygen
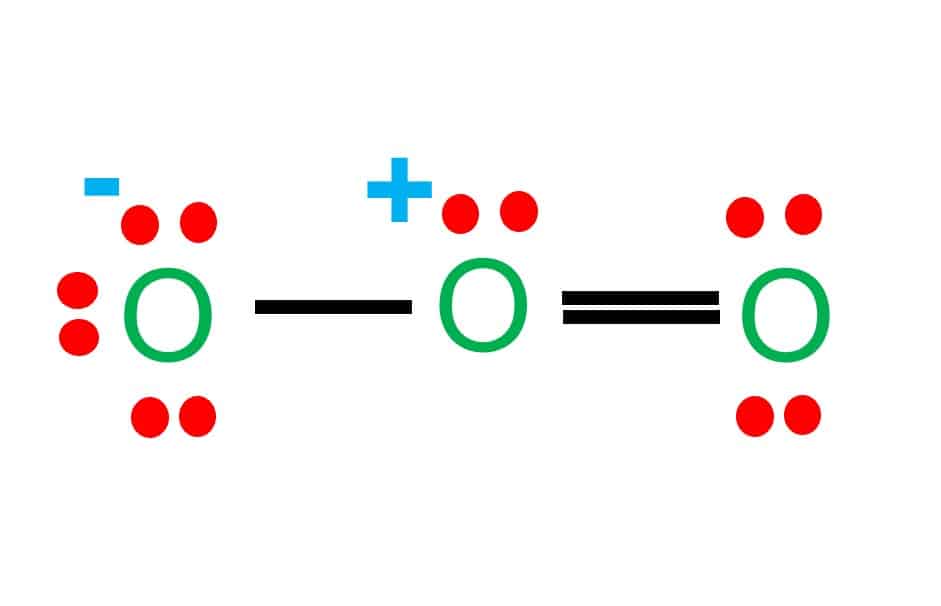
O3 Lewis Structure Step By Step Drawing What's Insight

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
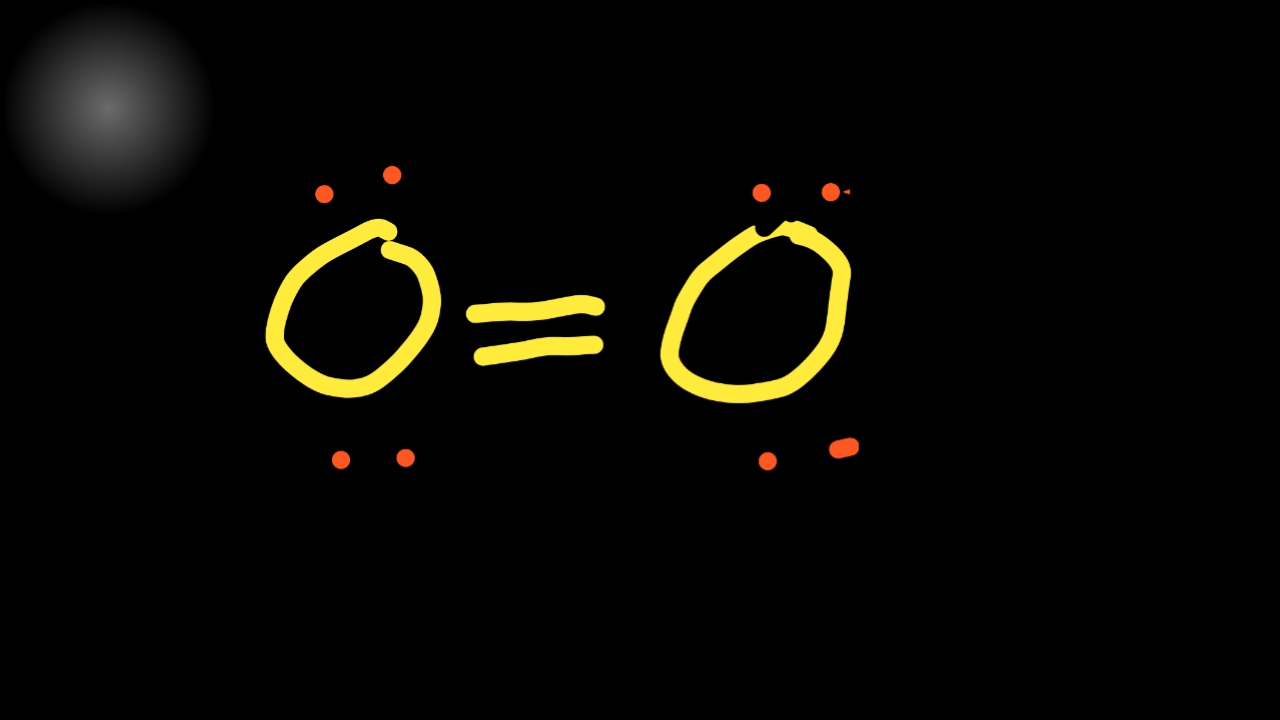
【2 Step】O2 Lewis StructureLewis Dot Structure for Oxygen(O,O2)Lewis

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration

Oxygen Atom Lewis Structure
Draw Lewis Structures Depicting The Bonding In Simple Molecules.
Write Lewis Symbols For Neutral Atoms And Ions.
Br Ch₂12 Cio Nh4 Sih4 H₂O* Give Detailed Solution With Structures, Don't….
Write Lewis Symbols For Neutral Atoms And Ions.
Related Post:
