Draw The Lewis Structure For A Hydrogen Molecule
Draw The Lewis Structure For A Hydrogen Molecule - By the end of this section, you will be able to: Try structures similar to h 2 o 2 (like hcl or ch 4) for more practice. Web if we can't get a satisfactory lewis structure by sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons. Web () you can use a drawing or use small beads or objects to represent electrons and work out the electron arrangement. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Draw lewis structures depicting the bonding in simple molecules. Web it is helpful if you: Mark atoms with lone pairs. Draw the lewis structure for a hydrogen (h2) molecule. Web the h 2 lewis structure is also one of the simpliest to draw. Draw a single bond between the two atoms. Draw the lewis structure for a hydrogen (h2) molecule. Web it is helpful if you: · 1 · jul 3 2018. Draw lewis structures depicting the bonding in simple molecules. The resulting lewis structure for h2 is: Place the two hydrogen atoms next to each other. If there are charges on atoms, mark them. Web in h 2 o, for example, there is a bonding pair of electrons between oxygen and each hydrogen. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. These electrons will usually be lone pairs. How to draw a lewis structure. Exceptions to the octet rule. Try structures similar to h 2 o 2 (like hcl or ch 4) for more practice. Answers to chapter 1 practice questions. · 1 · jul 3 2018. Web it is helpful if you: Answers to chapter 1 practice questions. If any electrons are left over, place them on the central atom. Web the h 2 lewis structure is also one of the simpliest to draw. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Exceptions to the octet rule. Consider formaldehyde (h 2 co) which contains 12 valence electrons. Answers to chapter 1 practice questions. Draw the lewis structure of the n 2 molecule. Figure 1.2e the lewis structure of a covalent bond between two oxygen atoms. Determine the total number of valence (outer shell) electrons. ∴ total available valence electrons = 1 + 7 = 8. Web hydrogen and halogen atoms tend to appear on the outside of the molecule and are rarely the central atom. Web lewis structures of simple molecules. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. If there are charges on atoms, mark them. Try to draw the h 2 o 2 lewis structure before watching the video. The sum of the valence electrons is 5 (from n) + 6 (from. Web hydrogen and halogen atoms tend to appear on the outside of the molecule and are rarely the central atom. Hydrogen atoms only need 2 valence electrons to have a full outer shell. Draw lewis structures depicting the bonding in simple molecules. Procedure for writing lewis structures of molecules. Use a different color or shape to represent the electrons of. Draw a single bond between the two atoms. The formula of this molecule suggests the following skeleton. Hydrogen atoms only need 2 valence electrons to have a full outer shell. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Draw lewis structures depicting the. If there are charges on atoms, mark them. Web the h 2 lewis structure is also one of the simpliest to draw. Using lewis structures to show valence electrons. Draw a skeletal structure connect the atoms to the central atom with a straight line representing a bond between the two atoms. Using the periodic table to draw lewis dot structures. 1.2.2 lewis structures of polyatomic molecules or ions. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web lewis structures of simple molecules. Web here are the steps to draw a lewis structure. These electrons will usually be lone pairs. These lewis symbols and lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. ∴ total available valence electrons = 1 + 7 = 8. For a molecule, we add the number of valence electrons (use the main group number) on each atom in the molecule. Sum the valence electrons from all the atoms. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). Figure 1.2d the lewis structure of covalent bond between hydrogen and chlorine. Move them around until all the atoms have 8 in their outer orbits. Draw the lewis structure of the n 2 molecule. Mark atoms with lone pairs. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. The sum of the valence electrons is 5 (from n) + 6 (from o) = 11.
Lewis structure Hydrogen Electron Symbol Chemical element, hydrogen
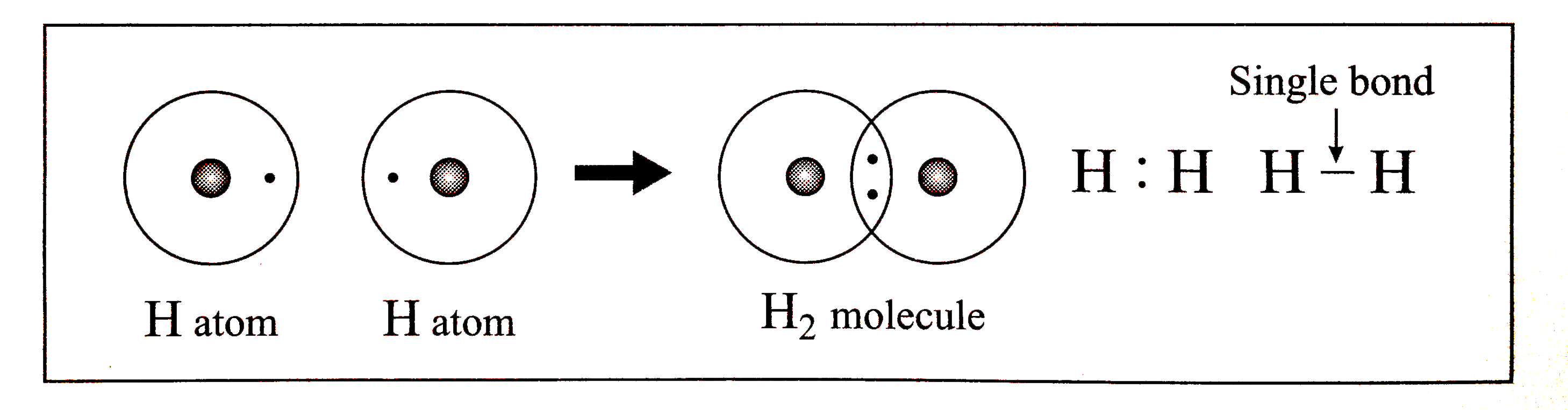
Draw the Lewis dot structure of hydrogen moelcule

Lewis Dot Diagram For Hydrogen

Lewis Dot Diagram For Hydrogen

H2 Lewis Structure How to Draw the Dot Structure for H2 YouTube

A stepbystep explanation of how to draw the H Lewis Dot Structure
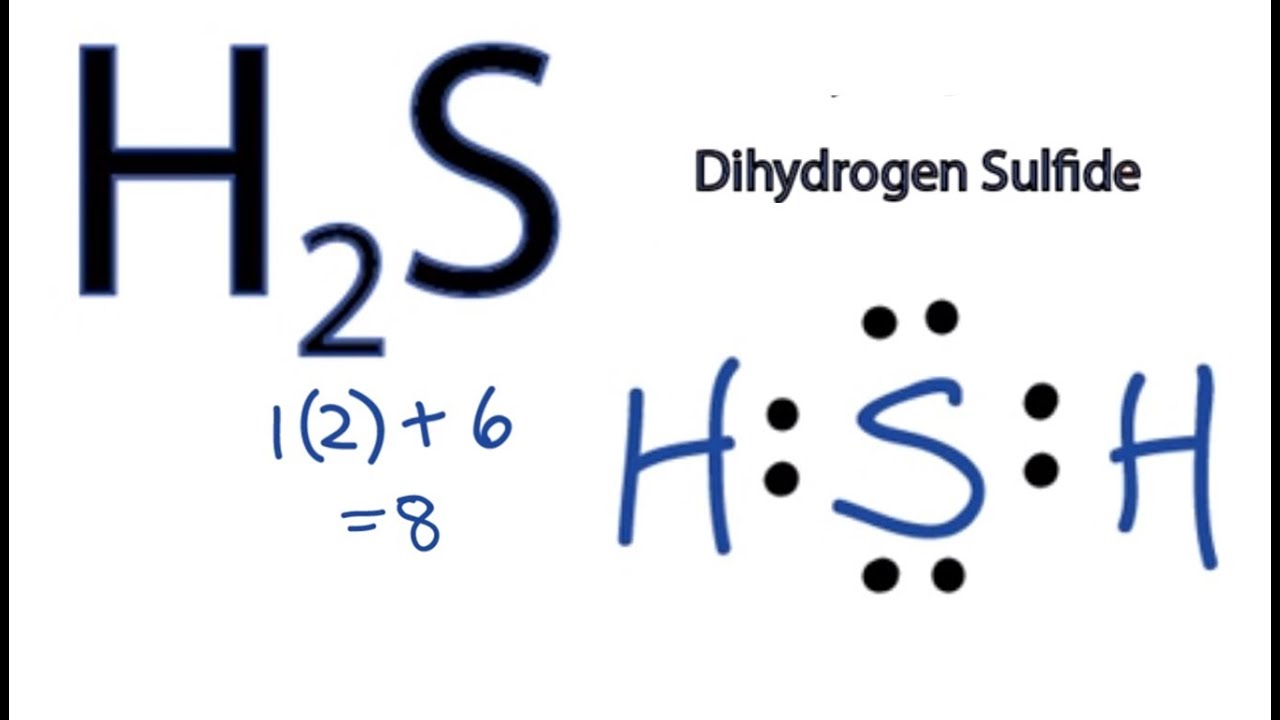
Estructura De Lewis H2s Estudiar

How to Draw a Lewis Structure

H2O Lewis Structure, Molecular Geometry, and Hybridization

H2O Lewis Structure, Molecular Geometry, and Hybridization
Place Two Electrons On Each Hydrogen Atom To Satisfy The Octet Rule.
A Lewis Structure Is A Diagram That Shows The Chemical Bonds Between Atoms In A Molecule And The Valence Electrons Or Lone Pairs Of Electrons.
This Problem Has Been Solved!
Watch The Video And See If You Missed Any Steps Or Information.
Related Post: