Draw The Lewis Dot Structure For H2O
Draw The Lewis Dot Structure For H2O - This is the lewis dot structure for h2o. Determine the total number of valence electrons in the molecule or ion. Look for the total valence electrons: Web here are the steps to draw a lewis structure. Be sure that you don't use more than the. The example is for the nitrate ion. What are some examples of lewis structures? Using lewis dot symbols to describe covalent bonding. Web lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. For h₂o, o must be the central atom. How to draw lewis structure for h 2 o; According to the octet rule, atoms will tend to lose, gain, or share electrons such that their valence electron shell resembles that of a noble gas. Web here, i have explained 6 simple steps to draw the lewis dot structure of h2o (along. Look for how many electrons are needed: For h₂o, o must be the central atom. O has 6 valence electrons, and each h has one. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Web lewis structure of water molecule contains two single bonds around oxygen atom. Web to use lewis dot symbols to explain the stoichiometry of a compound. Lewis dot structures are commonly referred to as electron dot structures or lewis structures. Web make sure you put the correct atom at the center of the water (h 2 o) molecule. Web lewis dot structures also called electron dot structures are diagrams that describe the chemical. Web here, i have explained 6 simple steps to draw the lewis dot structure of h2o (along with images). They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Web created by makethebrainhappy. Try to draw the h 2 o lewis structure before watching the video. In most cases, the noble. Web to use lewis dot symbols to explain the stoichiometry of a compound. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties.. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Using lewis dot symbols to describe covalent bonding. The video covers the basic lewis structures you'll. Be sure that you don't use more than the. Determine the total number of valence electrons in the molecule or ion. Web lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. By the end of this section, you will be able to: Lewis dot structures are commonly referred to as electron dot structures or lewis structures. Write lewis. Write lewis symbols for neutral atoms and ions. By the end of this section, you will be able to: Web here are the steps to draw a lewis structure. It is eight to form a single h2o molecule. According to the octet rule, atoms will tend to lose, gain, or share electrons such that their valence electron shell resembles that. How do you draw the lewis structure for ions? Web lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Web the lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready. You can find a procedure for drawing lewis structures at this location. Web make sure you put the correct atom at the center of the water (h 2 o) molecule. Lewis dot structures are commonly referred to as electron dot structures or lewis structures. It is eight to form a single h2o molecule. The example is for the nitrate ion. Web lewis structure of water molecule contains two single bonds around oxygen atom. Web make sure you put the correct atom at the center of the water (h 2 o) molecule. What is an example of a lewis structures practice problem? Web here, i have explained 6 simple steps to draw the lewis dot structure of h2o (along with images). So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Web the lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Web lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. It is four for one water (h2o) molecule according to the octet rule. Web what are lewis dot structures used for? It is eight to form a single h2o molecule. Web lewis dot structure for h2o (water) it is helpful if you: I also go over hybridization,. According to the octet rule, atoms will tend to lose, gain, or share electrons such that their valence electron shell resembles that of a noble gas. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: For the h2o structure use the periodic table to find the total number of.
H2O Lewis Structure, Molecular Geometry, and Hybridization

Draw Step By Step The Lewis Structure For Water (H2O)
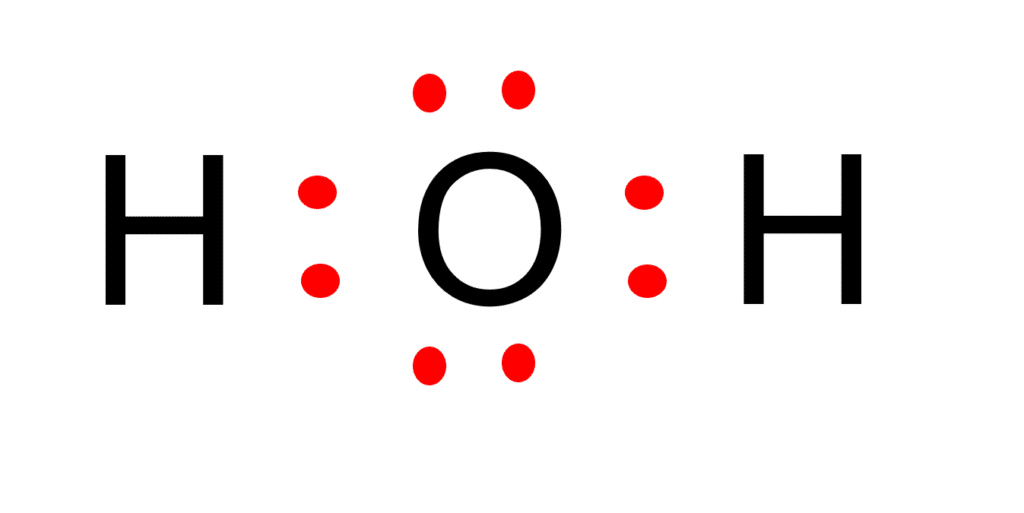
Lewis Structures Hydrogen (H2), and Water (H2O) What's Insight
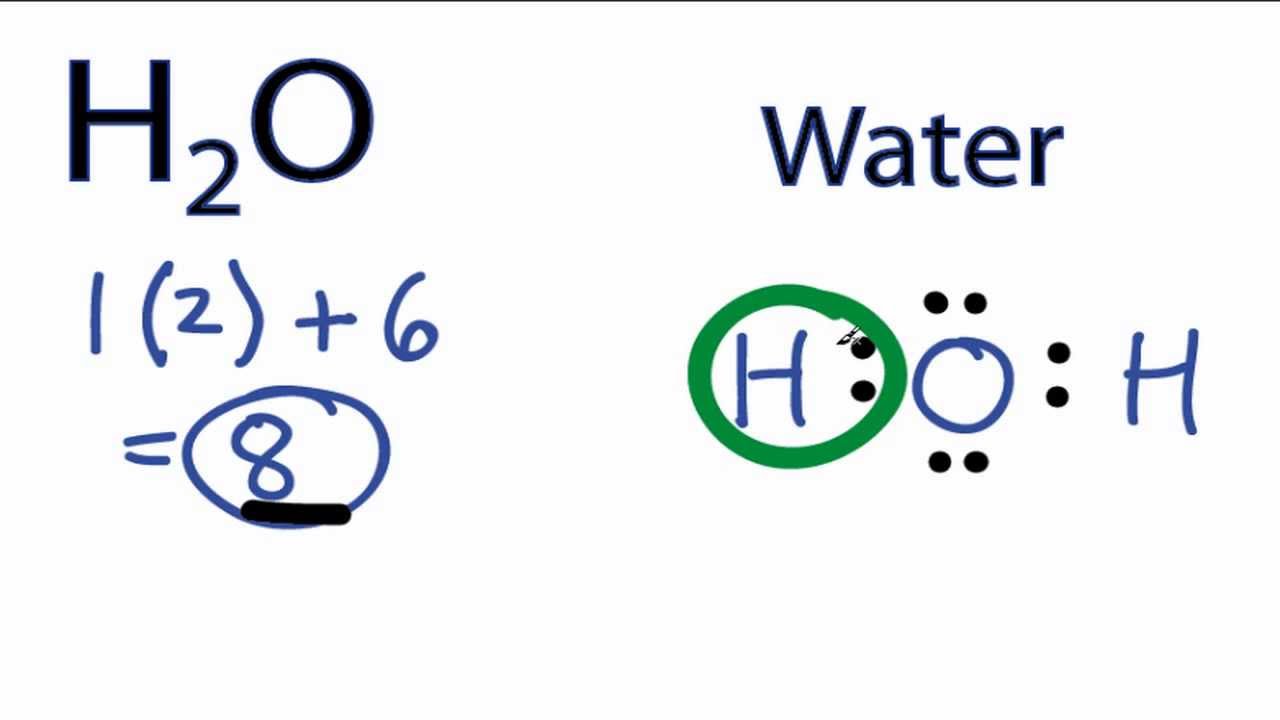
Draw The Lewis Structure Of H2O
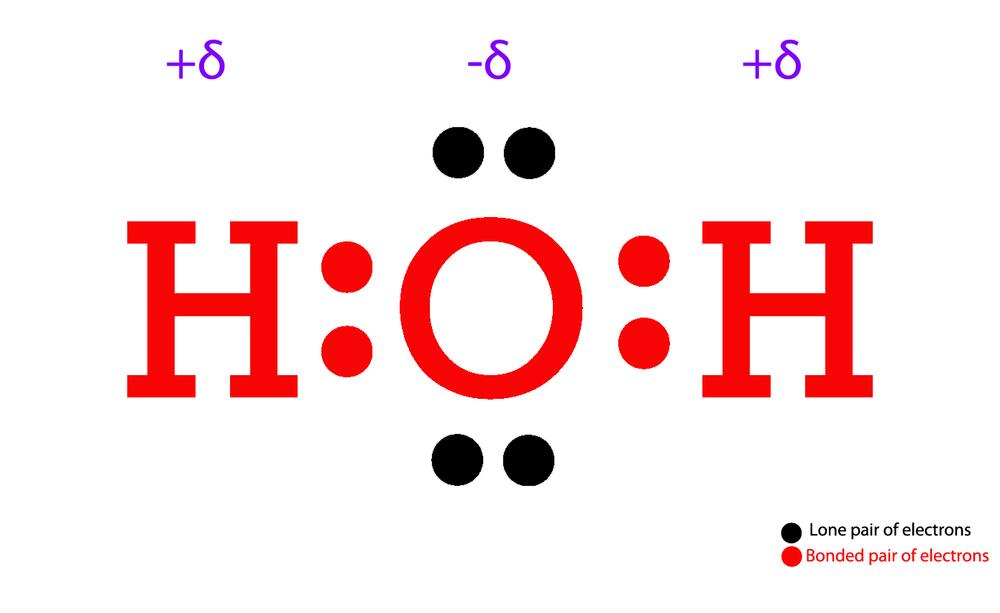
Draw The Lewis Structure Of H2o

H2O Lewis structure and Molecular Geometry [No1 Best Explanation

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
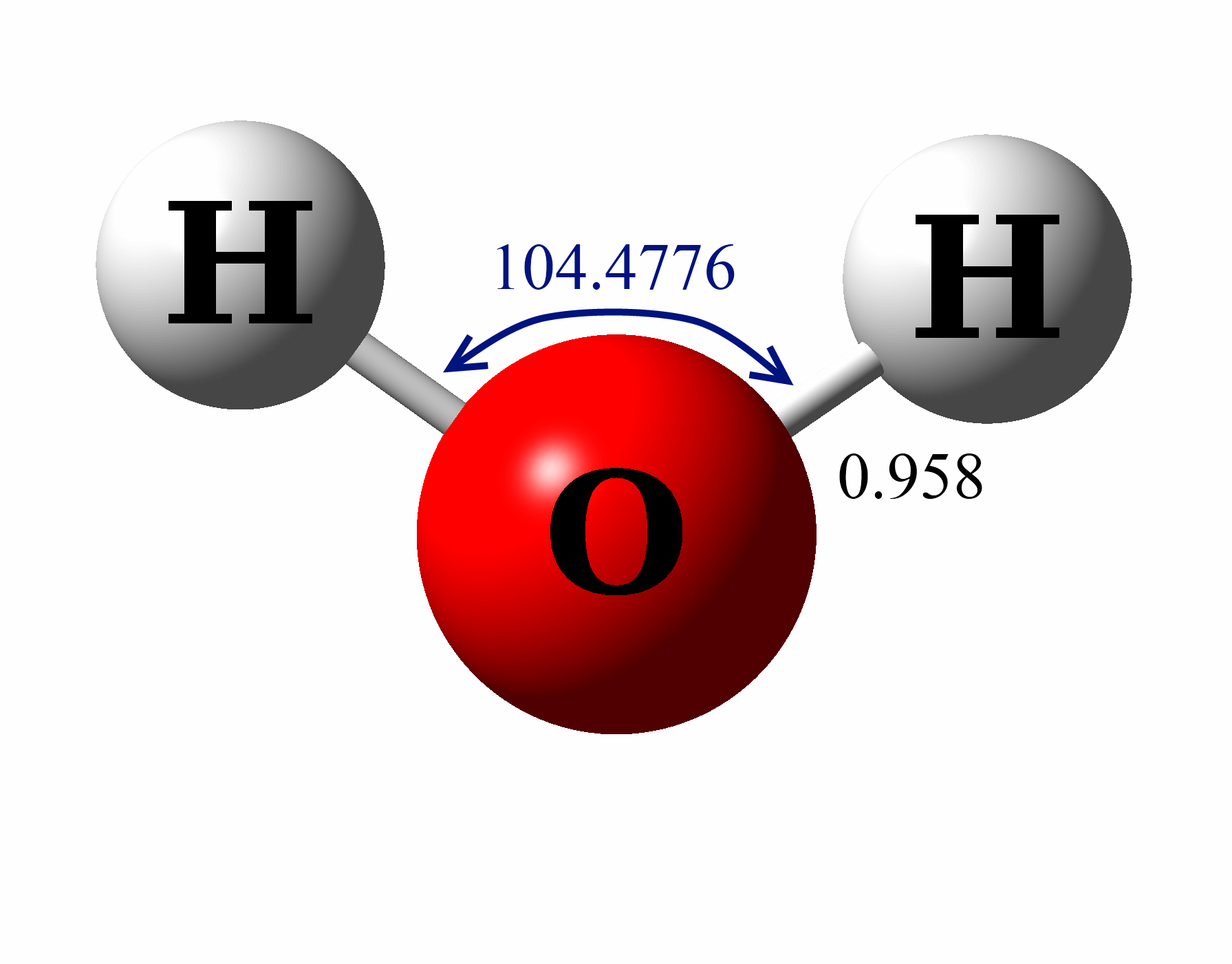
Estrutura De Lewis H2o

In this video we are going to learn about the Lewis structure of H2O

H2O Lewis Structure, Molecular Geometry, and Hybridization
Look For The Total Valence Electrons:
Look For How Many Electrons Are Needed:
A Lewis Structure Is A Diagram That Shows The Chemical Bonds Between Atoms In A Molecule And The Valence Electrons Or Lone Pairs Of Electrons.
With The Lewis Structure For Water (H 2 O) Remember That Water Only Needs Two Valence Electrons To Have A Full Outer Shell.
Related Post: