Draw The Lewis Dot Structure For Ca
Draw The Lewis Dot Structure For Ca - The lewis symbol for carbon: For the ca2+ structure use the periodic table to find the total. The element symbol, together with the dots, is called the lewis symbol. Lewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which are represented as dots. Web to use the lewis structure calculator follow these steps: The lewis dot structure for calcium as an ion is ca2+ with no dots around it, as it has lost all valence electrons. The number of dots equals. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Show the formal charges of all atoms in the correct structure. Web there are multiple ways to draw lewis dot structures. This type of symbolic representation can help describe compound formation, especially for. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. You start by counting the number of electrons the atoms donate. Web how to draw the lewis dot structure for cas : Web key concepts and summary. The number of dots equals the number of valence electrons in the atom. You start by counting the number of electrons the atoms donate to the structure, and you need to take into account charge if you are drawing an ion. Web a lewis. Web there are multiple ways to draw lewis dot structures. We can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. Dots representing the lone pairs. Write lewis symbols for neutral atoms and ions. Web to use the lewis structure calculator follow these steps: Ammonium chloride, nh4cl, is a very soluble salt in water. Ch3oo (unpaired electron on terminal oxygen) question 110. Write lewis symbols for neutral atoms and ions. (a) draw the lewis structures of the ammonium and chloride ions. Lewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which are represented as dots. 88k views 5 years ago. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. 40k views 5 years ago. Web there are multiple ways to draw lewis dot structures. I show you where calcium is on the periodic table and how to determine how. Dots representing the lone pairs. Web lewis structures are visual representations of the bonds between atoms and illustrate the lone pairs of electrons in molecules. Each of the four valence electrons is represented as a dot. Web for example, the lewis symbol of carbon depicts a “c’ surrounded by 4 valence electrons because carbon has an electron configuration of 1s. How to draw the ca2+ lewis dot structure. Web draw lewis structures for each free radical implicated in this theory of aging. Web draw the lewis dot structure of a given molecule or ion. Dots representing the lone pairs. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. This type of symbolic representation can help describe compound formation, especially for. We will follow a flow chart that allows you to identify exceptions to octet rules and resonance structures. In this case, we can condense the last few steps, since not all of them apply. Lewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which. Web to use the lewis structure calculator follow these steps: Web key concepts and summary. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. The number of dots equals the number of valence electrons in the atom. In all cases, these bonds involve the. They can also be called lewis dot diagrams and are used as a simple way to show the configuration of atoms within a molecule. Web key concepts and summary. Give examples for molecules and ions that do not follow the octet rule. Ammonium chloride, nh4cl, is a very soluble salt in water. The student's question involves drawing the lewis dot. Write lewis symbols for neutral atoms and ions. Ammonium chloride, nh4cl, is a very soluble salt in water. 117k views 9 years ago. Thus far, we have discussed the various types of bonds that form between atoms and/or ions. In this case, we can condense the last few steps, since not all of them apply. The student's question involves drawing the lewis dot structure for the element calcium (ca) and showing the formal charges of all atoms in the structure. Lewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which are represented as dots. Ca draw the lewis dot structure for ca. Ch3oo (unpaired electron on terminal oxygen) question 110. For the ca2+ structure use the periodic table to find the total. The formal charge of the calcium ion is +2. Electrons that are not in the valence level are not shown in the lewis symbol. For calcium iodide, an ionic solid, we conceive of ca2+, and 2 × i − ions. (a) draw the lewis structures of the ammonium and chloride ions. Web the calcium lewis dot structure representation only shows the presence of 2 electrons around the calcium atom. We will follow a flow chart that allows you to identify exceptions to octet rules and resonance structures.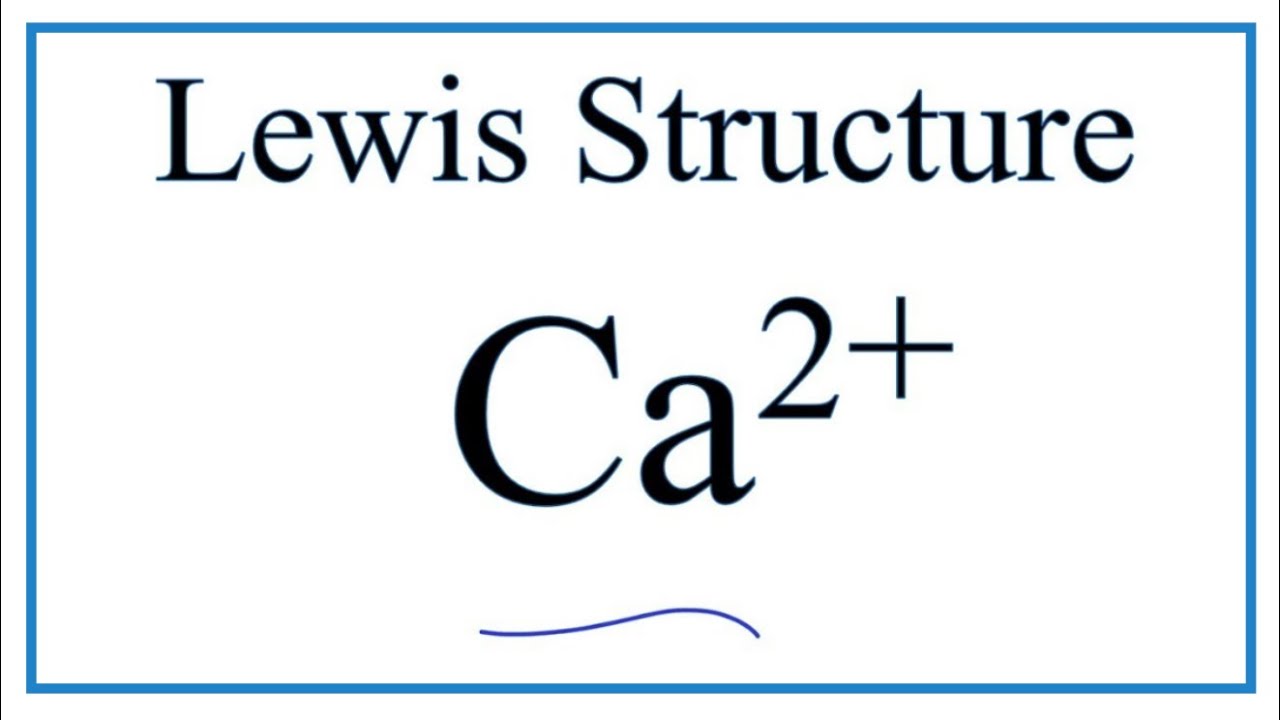
How to draw the Ca2+ Lewis Dot Structure. YouTube

Draw the Lewis Structure of CaCl2 (calcium chloride) YouTube

How To Draw Lewis Structures A Step By Step Tutorial

Comment dessiner une représentation de Lewis Wiki Chimie

3 Ways to Draw Lewis Dot Structures wikiHow

whats the lewis dot diagram for calcium

Draw the Lewis Structure of Calcium Iodide (CaI2) YouTube

Draw the Lewis Structure of Ca3P2 (calcium phosphide) YouTube

CaCl2 Lewis Structure (Calcium Chloride) YouTube
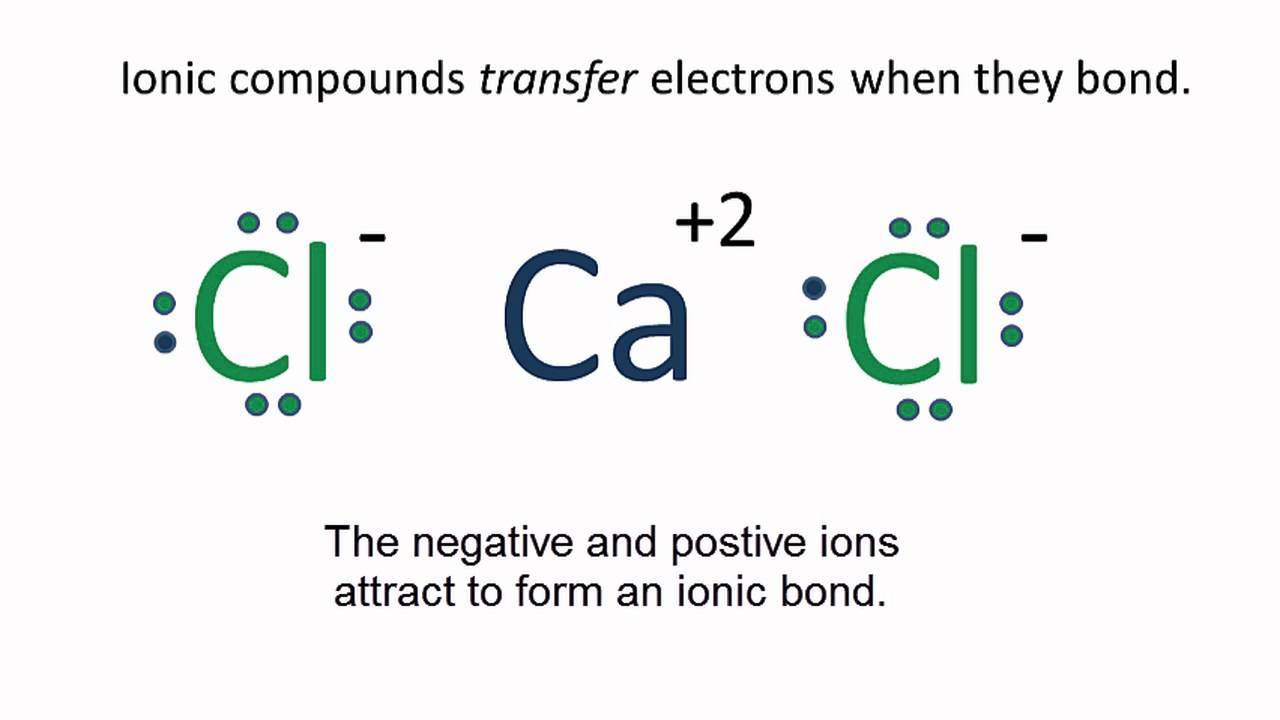
CaCl2 Lewis Structure How to draw the Lewis Dot Structure for Calcium
Give Examples For Molecules And Ions That Do Not Follow The Octet Rule.
40K Views 5 Years Ago.
We Can Draw The Lewis Structure Of Any Covalent Molecule By Following The Six Steps Discussed Earlier.
Web There Are Multiple Ways To Draw Lewis Dot Structures.
Related Post: