Draw The Electron Dot Formula For The Element Sulfur
Draw The Electron Dot Formula For The Element Sulfur - That's the formal charge for sulfur. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Web remember each bond is 2 electrons, and each lone pair is 2 electrons. Web sulfide, any of three classes of chemical compounds containing the element sulfur. This chapter will explore yet another shorthand method of representing the valence electrons. To draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. The number of dots equals. Web 1 lesson objectives. Include all of the valence electrons. The periodic table shows you how many valence electron each element has. Calculate the number of valence electrons: Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons. Web formal charge = 7 − [2 + 1/2 × 10] = 0. Calculate the formal charge on each of the oxygen (o) atoms labeled a, b, and c in the. Calculate the formal charge on each of the oxygen (o) atoms labeled a, b, and c in the following lewis structure. 8 + (6 × × 7) = 50; Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Remember that formal charge is calculated by taking the # of valence electrons, minus the lone electrons. 8 + (2 × × 7) = 22 xef 6: 3 x 6 = 18 valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For representative elements, the number of valence electrons equals the group number on the periodic table. Web draw a lewis electron dot diagram for an atom. Include all of the valence electrons. You can add the valence electrons by clicking on the. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in. Calculate the. You can add the valence electrons by clicking on the. Draw a skeleton joining the atoms by single bonds. A= double bond on the right. Web sulfide, any of three classes of chemical compounds containing the element sulfur. In this case, we can condense the last few steps, since not all of them apply. There are four covalent bonds in the skeleton structure for sf 4. Sulfur, which is located in group 6a, has 6 valence electrons. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.. Due to the small and highly electronegative nature of fluorine, the oxyacids of the this element are much less. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web remember each bond is 2 electrons, and each lone pair is 2 electrons. Web sulfide, any of three. 82k views 10 years ago. In this case, we can condense the last few steps, since not all of them apply. Web sulfide, any of three classes of chemical compounds containing the element sulfur. H 2 cch 2 f. For representative elements, the number of valence electrons equals the group number on the periodic table. Added jun 9, 2014 by webtester in chemistry. To draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web sulfur has 6 valence electrons if you look at the periodic table. Send feedback | visit wolfram|alpha. A= double bond on the right. For fluorine on the periodic table, it has 7 valence electrons. C= single bond on the left. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. 3 all. Know the importance of lewis dot in bonding. That's the formal charge for sulfur. Calculate the formal charge on each of the oxygen (o) atoms labeled a, b, and c in the following lewis structure. 6 + 18 + 2 extra = 26 valence electrons. Consider the lewis structure for sulfur tetrafluoride (sf 4) which contains 34 valence electrons. Find more chemistry widgets in wolfram|alpha. This problem has been solved! Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Sulfur, which is located in group 6a, has 6 valence electrons. Web draw a lewis electron dot diagram for an atom. Draw a skeleton joining the atoms by single bonds. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Include all of the valence electrons. Web sulfur has 6 valence electrons if you look at the periodic table. You can add the valence electrons by clicking on the. 6 + 4(7) = 34.
SOLVED Draw the electrondot formula for the element sulfur. You can

Dot Diagram For Sulfur
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
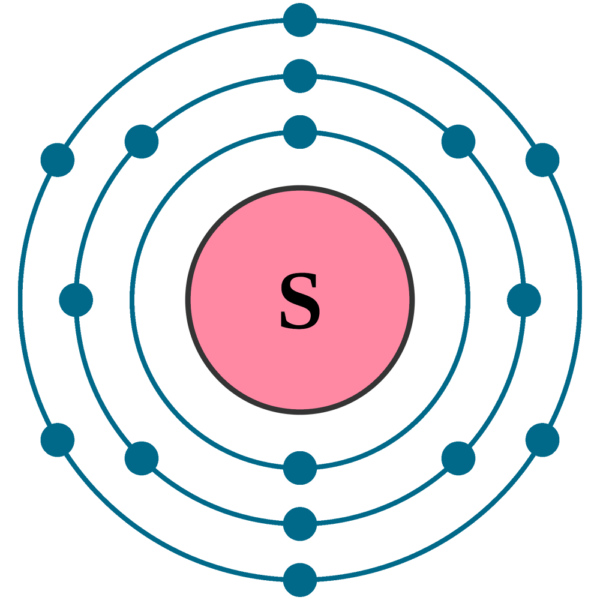
Atom Diagrams Electron Configurations of the Elements
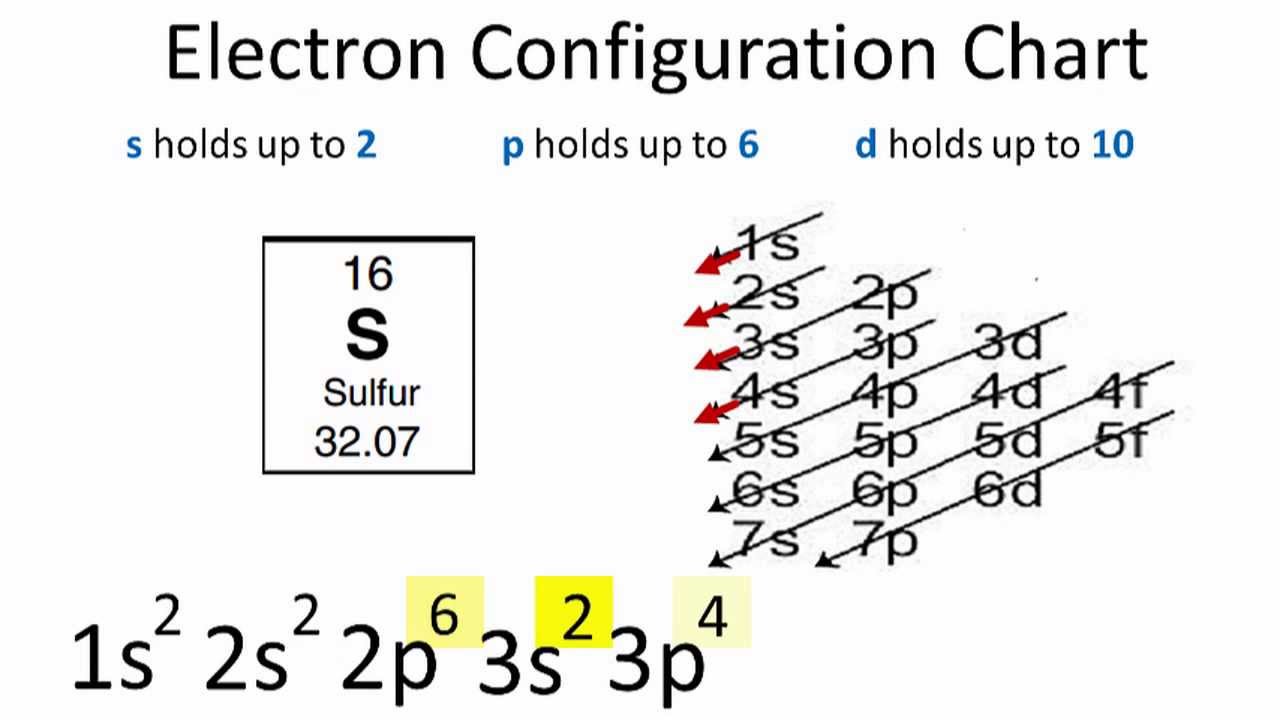
Sulfur Electron Configuration (S) with Orbital Diagram

Diagram representation of the element sulfur Vector Image
Sulfur S (Element 16) of Periodic Table Elements FlashCards
Solved Draw the electrondot formula for the element

Draw the electrondot formula for the element sulphur YouTube

Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist

Sulfur Definition, Facts, Symbol, Allotropes, Properties, Uses
I Show You Where Sulfur Is On The.
Web Lewis Structures (Also Known As Lewis Dot Diagrams, Electron Dot Diagrams,Lewis Dot Formula Lewis Dot Structures, And Electron Dot Structures) Are Diagrams That Show The Bonding Between Atoms Of A Molecule And The Lone Pairs Of Electrons That May Exist In.
Send Feedback | Visit Wolfram|Alpha.
Because This Requires Using Eight Valence Electrons To Form The Covalent Bonds That Hold The Molecule Together, There Are 26 Nonbonding Valence Electrons.
Related Post: