Draw The Electron Configuration For A Neutral Atom Of Sulfur
Draw The Electron Configuration For A Neutral Atom Of Sulfur - This means that it has a total of 16 electrons. Here’s the best way to solve it. Web for hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure \(\pageindex{1}\)), and the electron configuration is written as. Web the electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. Web the electron configuration for a sulfur atom is [ne]3s23p4 and its lewis symbol is shown in the figure below. Electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. Web sulfur has an atomic number of 16. Web intro to electron configurations; The electron configuration for a neutral atom of sulfur is 1s² 2s². Web an electron configuration diagram is a model that depicts the. So that means a sulfur atom has 16 protons. Using only the periodic table; Web what is the electron configuration of a sulfur atom? Explain it to a child. Web looking at the periodic table, we see that the atomic number for sulfur is 16. So that means a sulfur atom has 16 protons. Web looking at the periodic table, we see that the atomic number for sulfur is 16. Using only the periodic table; Electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. Electron configurations list every subshell for an. Using only the periodic table; Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. In order to maximize their stability, electrons will always prefer to occupy the lowest energy orbital. And if it's a neutral atom it would have 16 electrons. Electronic structure drawing a box diagram of the electron configuration. Web what is the electron configuration of a sulfur atom? Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the. The electron configuration for a neutral atom of sulfur is 1s² 2s². Web for hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in. Explain it to a child. Web sulfur has an atomic number of 16. Using only the periodic table; And if it's a neutral atom it would have 16 electrons. Web draw the electron configuration for a neutral atom of sulfur. Therefore, there are only 6. And if it's a neutral atom it would have 16 electrons. Subshells are described by writing the. This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p. Electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Using only the periodic table; This means that it has a total of 16 electrons. Web what is the electron configuration of a sulfur atom? In order to maximize their stability, electrons will always prefer to occupy the lowest energy orbital. Using the aufbau principle, the pauli exclusion principle, and hund's rule to predict an atom's electron configuration using the. Web sulfur has an atomic number of 16. And if it's a neutral atom it would have 16 electrons. The electron configuration for a neutral atom of sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴. This means that sulfur has two electrons. The noble gas closest to it is neon, with an atomic number of 10. And if it's a neutral atom it would have 16 electrons. Here’s the best way to solve it. This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p. Using only the periodic table; What is the valence shell electron configuration of a. Therefore, there are only 6. Web looking at the periodic table, we see that the atomic number for sulfur is 16. Web the electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. The electron configuration for a neutral atom of sulfur is 1s² 2s². Subshells are described by writing the. Electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. Web what is the electron configuration of: Web the electron configuration for a sulfur atom is [ne]3s23p4 and its lewis symbol is shown in the figure below. Web what is the electron configuration of a sulfur atom? Therefore, there are only 6. Web sulfur has an atomic number of 16. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Web the electron configuration of a neutral sulfur atom will thus be s: What is the valence shell electron configuration of a calcium atom? This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p. Web an electron configuration diagram is a model that depicts the. Using only the periodic table; Here’s the best way to solve it. Web the electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. Web the electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4.
Draw The Electron Configuration For A Neutral Atom, HD Png Download
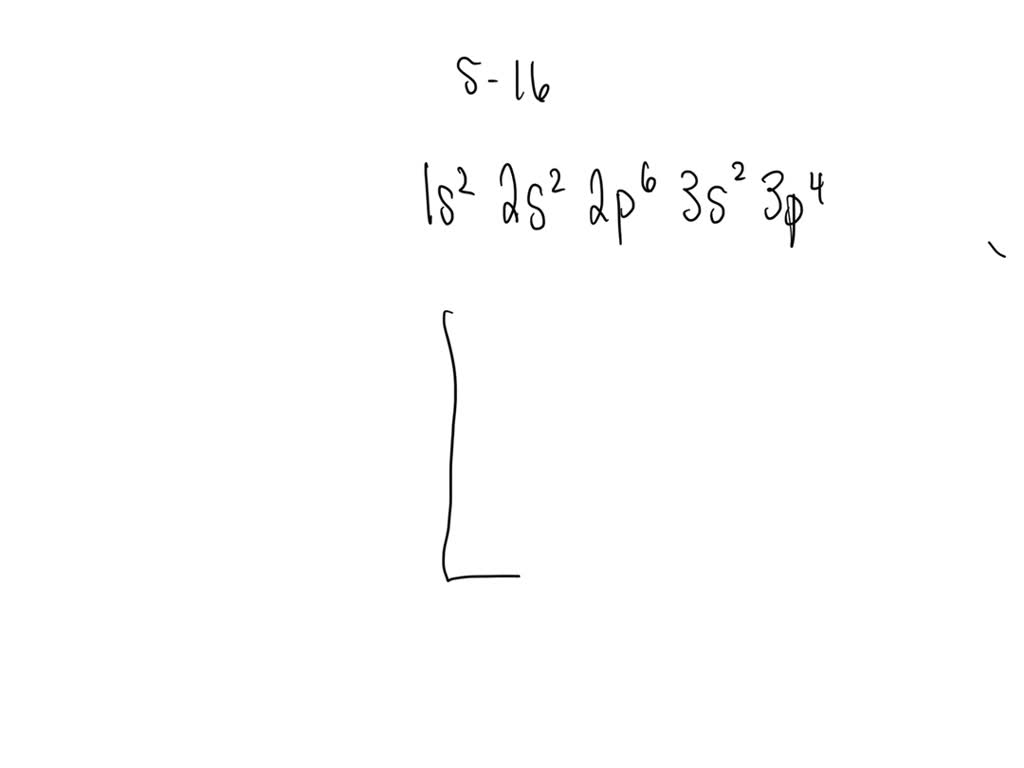
SOLVED Draw the electron configuration for a neutral atom of sulfur.
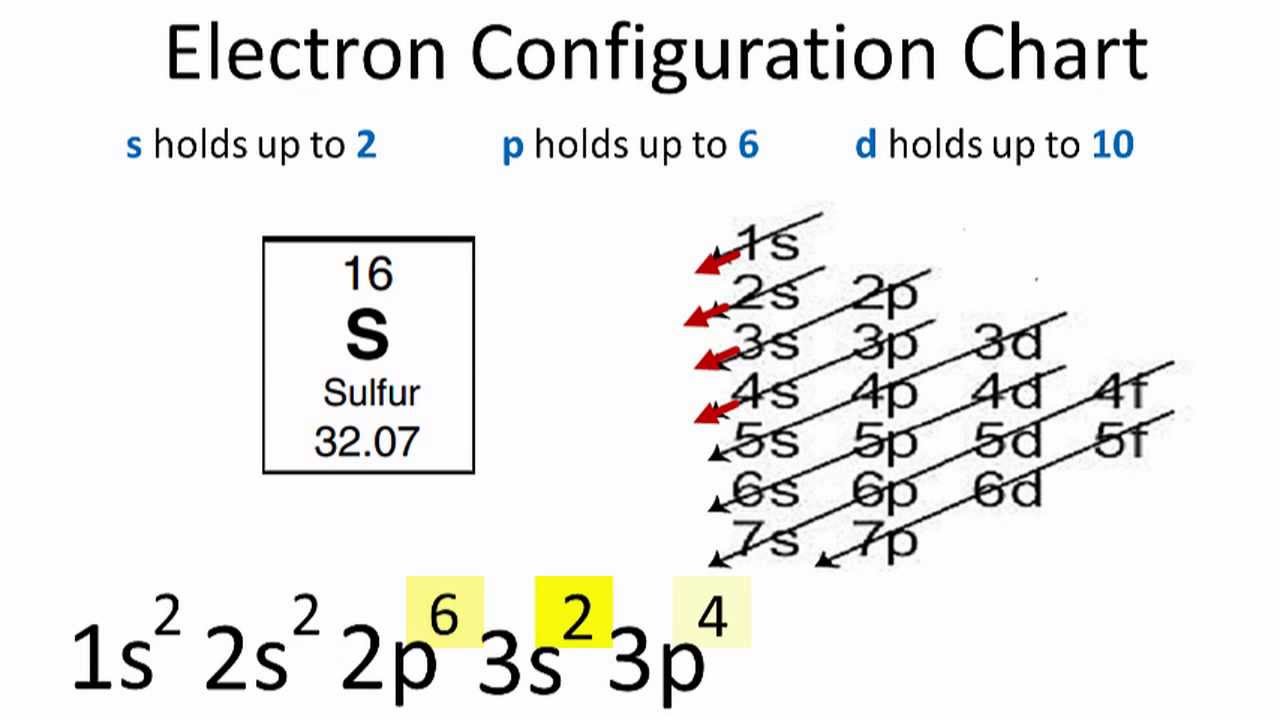
Sulfur Electron Configuration YouTube
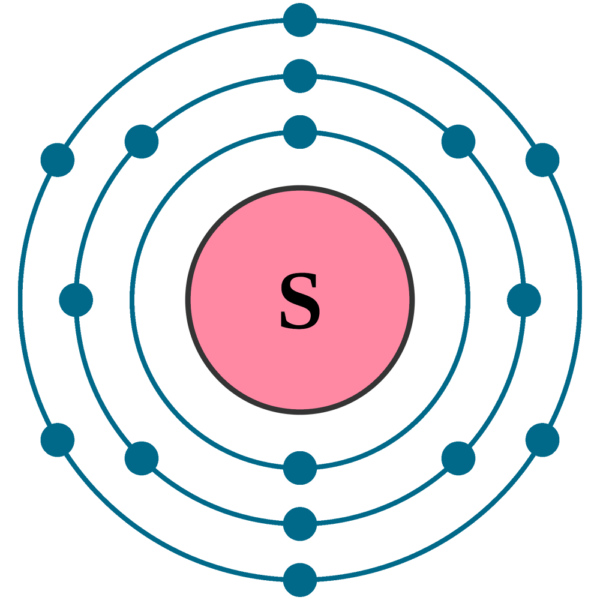
Sulfur S (Element 16) of Periodic Table Elements FlashCards
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
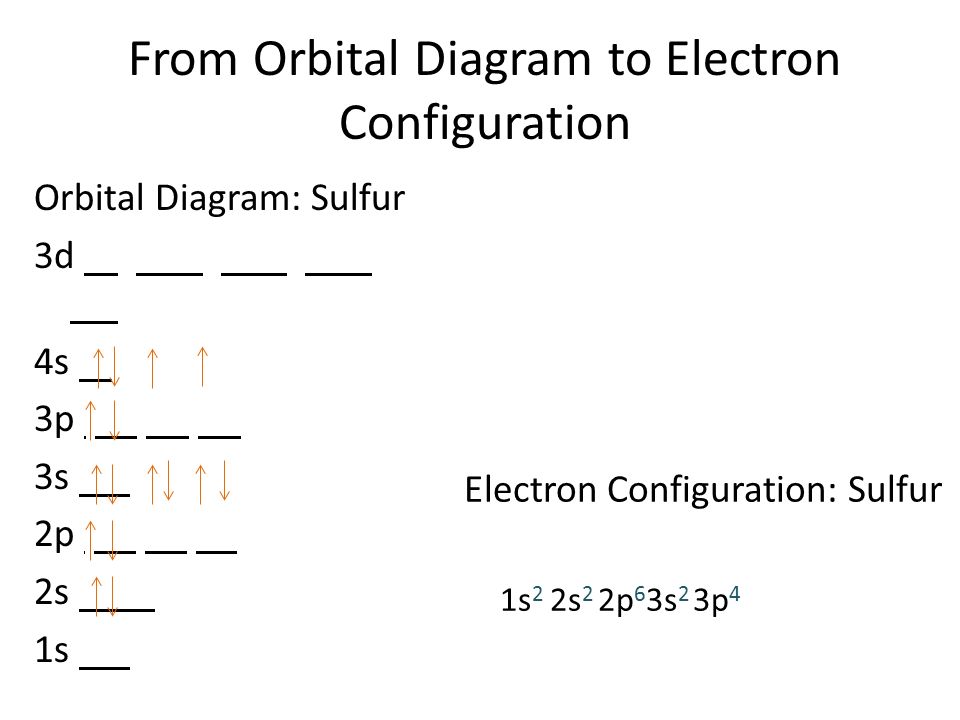
Sulfur Electron Configuration (S) with Orbital Diagram
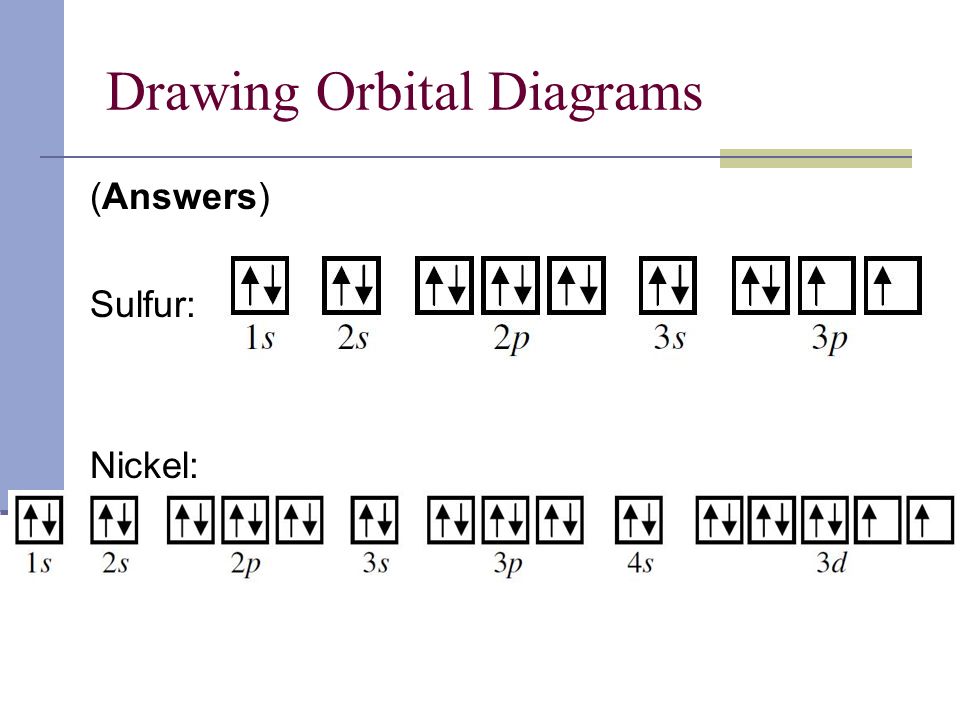
Orbital Box Diagram For Sulfur

Diagram representation of the element sulfur Vector Image
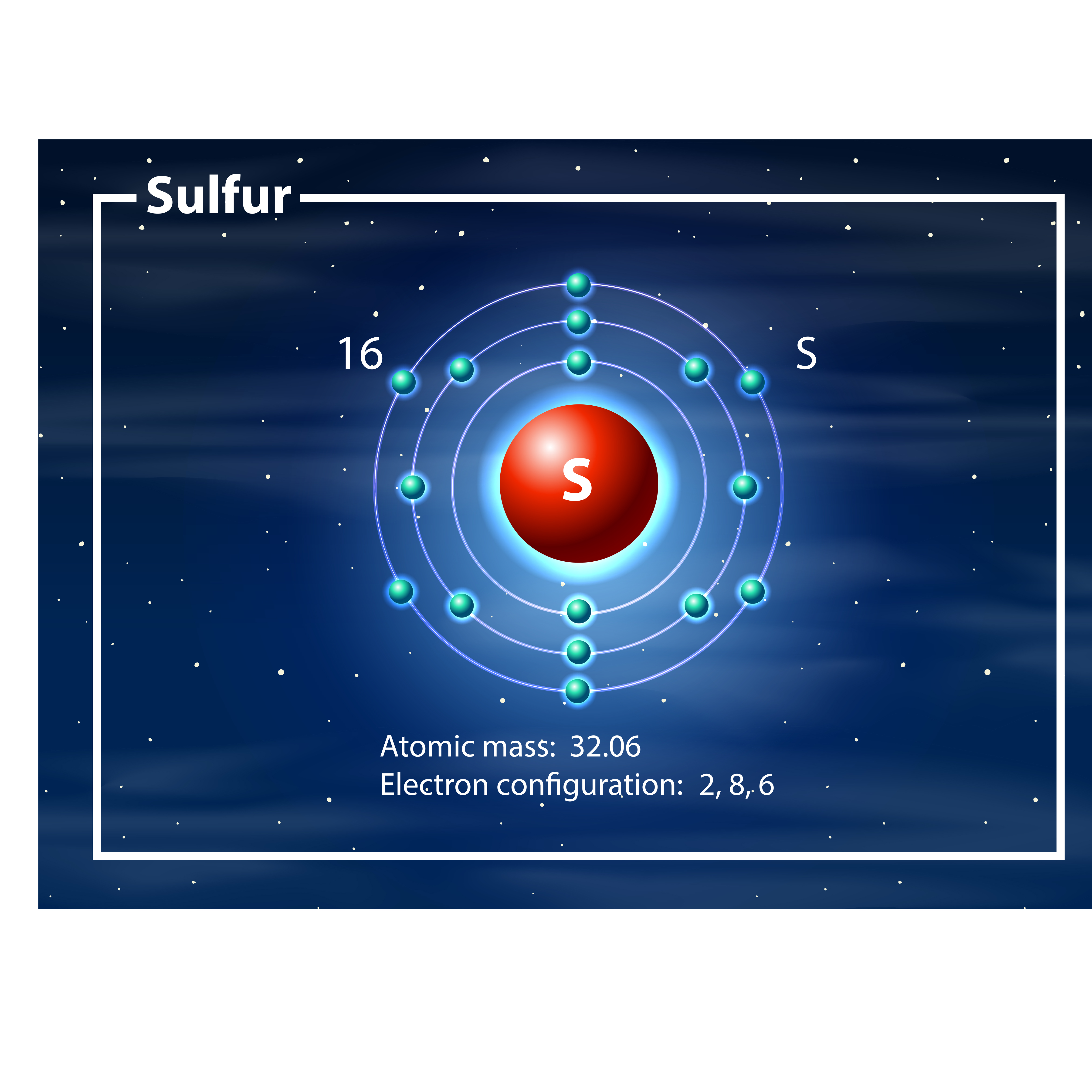
Electron Configuration Of Sulfur

Sulfur Atom Science Notes and Projects
The Noble Gas Closest To It Is Neon, With An Atomic Number Of 10.
So That Means A Sulfur Atom Has 16 Protons.
This Means That It Has A Total Of 16 Electrons.
Web Intro To Electron Configurations;
Related Post: