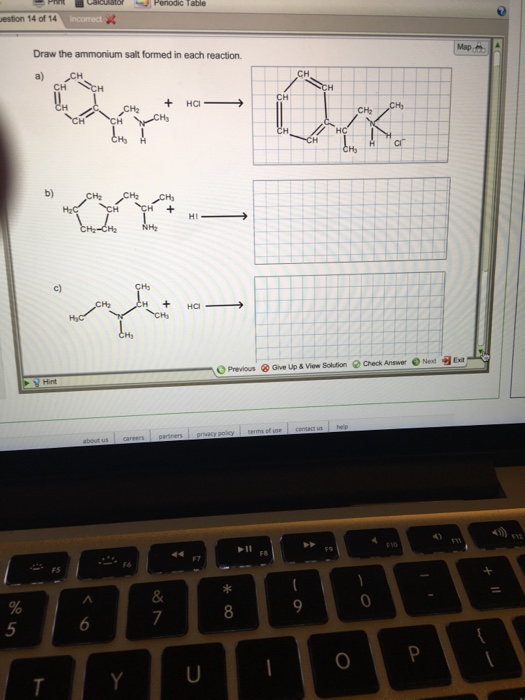
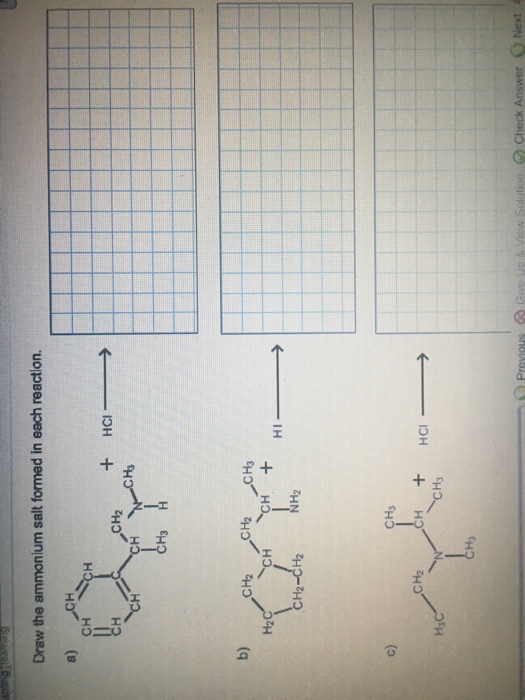
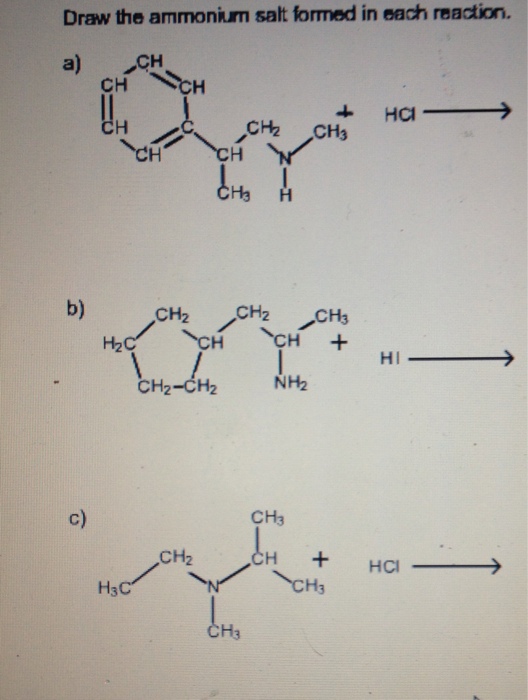
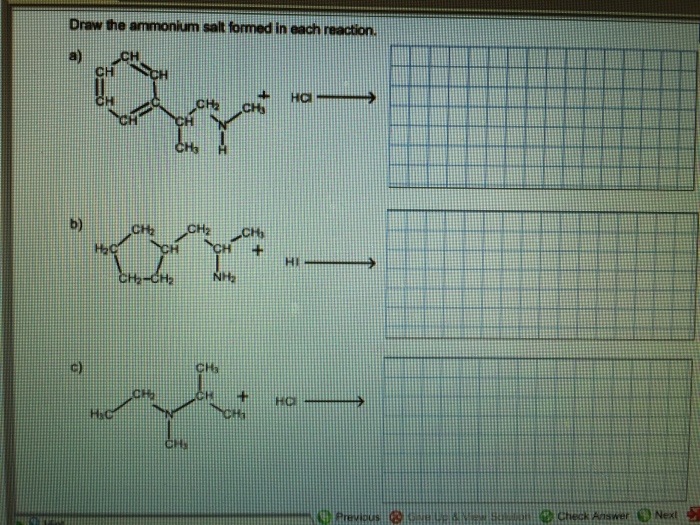
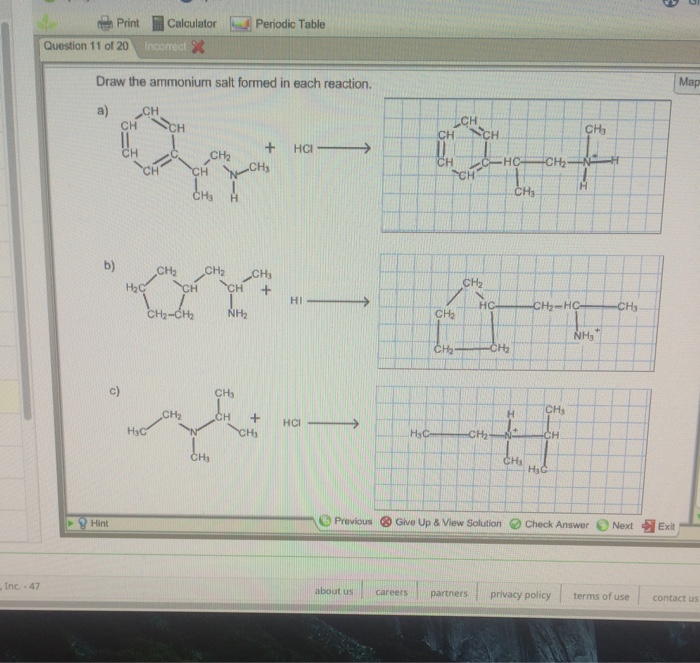
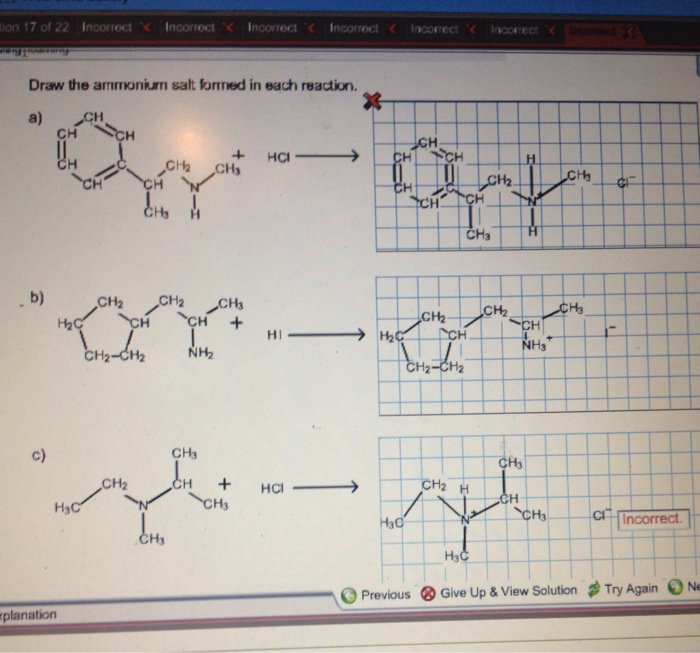
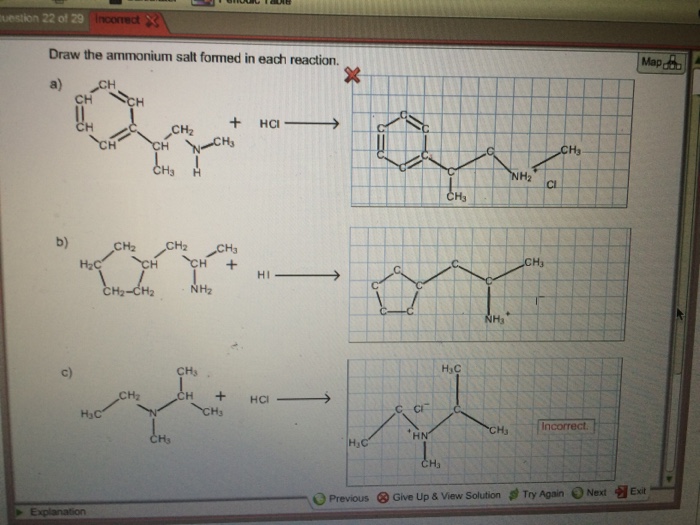
Draw The Ammonium Salt Formed In Each Reaction
Draw The Ammonium Salt Formed In Each Reaction - We want to give a balanced reaction to the problem. Web the ammonium ion is the conjugate acid of the base ammonia, nh 3; Select draw rings more erase h ci hci n. Amines with low water solubility can react with acids to form water soluble ammonium. It breaks into ion when we say so. Web the basicity of amines is determined by the reactivity of the lone pair on the nitrogen atom. Draw the ammonium salt formed in each reaction. Reaction a modify the structure to give the product of reaction a. This is where we're looking at ammonium chloride. Its acid ionization (or acid hydrolysis) reaction is represented by nh 4 + ( a q ) + h 2 o ( l ) ⇌ h 3 o + ( a q ) + nh 3 ( a q ) k a = k w / k b nh 4 + ( a q ) + h 2 o ( l ) ⇌ h 3. We have to draw the ammonia salt formed in this case now. Nitrogen and a pair of electrons are present here. Step 1/2first, we need to write the chemical formula for ammonium ion, which is $\mathrm {nh_4^+}$.answernext, we need to consider the reaction. Modify the structure to give the product of reaction a. Reaction a modify the structure to give. We have to draw the ammonia salt formed in this case now. Making secondary amines and their salts in the first stage of the reaction, you get the salt of a secondary amine formed. Web draw the condensed structural formula for the ammonium salt obtained when each of the amines in problem ( write a balanced chemical equation for the. Web the products of the reactions include secondary and tertiary amines and their salts, and quaternary ammonium salts. Its acid ionization (or acid hydrolysis) reaction is represented by nh 4 + ( a q ) + h 2 o ( l ) ⇌ h 3 o + ( a q ) + nh 3 ( a q ) k a. Its acid ionization (or acid hydrolysis) reaction is represented by nh 4 + ( a q ) + h 2 o ( l ) ⇌ h 3 o + ( a q ) + nh 3 ( a q ) k a = k w / k b nh 4 + ( a q ) + h 2 o (. Making secondary amines and their salts in the first stage. Draw the ammonium salt formed in each reaction. We want to give a balanced reaction to the problem. Modify the structure to give the product of reaction a. Hydrochloric acid is a polar molecule, h plus and. Ch 3 nhch 3 (aq) + hno 3 (aq) → We have to draw the ammonia salt formed in this case now. Select draw rings more erase h ci hci n. Web draw the ammonium salt formed in each reaction. Web what salt is formed in each reaction? Ch 3 nh 2 (aq) + hbr(aq) →; Draw the ammonium salt formed in each reaction. Amines, amides, anilines, imines, and nitriles, the central question is: Reaction a modify the structure to give the product of reaction a. Draw the ammonium salt formed in each reaction. Reaction a modify the structure to give the product of reaction a. Web the ammonium ion is the conjugate acid of the base ammonia, nh 3; Or hci incorrect reaction b draw the product of reaction b. Hydrochloric acid is a polar molecule, h plus and. All atoms should be drawn reaction a modify the structure to give the product. Amines, amides, anilines, imines, and nitriles, the central question is: Modify the structure to give the product of reaction a. (a) $\mathrm{nhch}_{3}$ (b) $\mathrm{ch}_{3} \mat… 01:31 draw the. Most applications involving this class of compounds are eliminations, but a few examples of s n 2 substitution have been reported. Its acid ionization (or acid hydrolysis) reaction is represented by nh. Web what salt is formed in each reaction? Its acid ionization (or acid hydrolysis) reaction is represented by nh 4 + ( a q ) + h 2 o ( l ) ⇌ h 3 o + ( a q ) + nh 3 ( a q ) k a = k w / k b nh 4 + (. Web draw the ammonium salt formed in each reaction. Draw the ammonium salt formed in each reaction. Ch 3 nhch 3 (aq) + hno 3 (aq) → Web draw the ammonium salt formed in each reaction: Step 1/2first, we need to write the chemical formula for ammonium ion, which is $\mathrm {nh_4^+}$.answernext, we need to consider the reaction. Amines with low water solubility can react with acids to form water soluble ammonium. Making secondary amines and their salts in the first stage of the reaction, you get the salt of a secondary amine formed. Reaction a draw the product of reaction a. Web the basicity of amines is determined by the reactivity of the lone pair on the nitrogen atom. All atoms should be drawn. Draw the ammonium salt formed in each reaction. Ch 3 nh 2 (aq) + hbr(aq) →; All atoms should be drawn. (a) $\mathrm{nhch}_{3}$ (b) $\mathrm{ch}_{3} \mat… 01:31 draw the. Web the products of the reactions include secondary and tertiary amines and their salts, and quaternary ammonium salts. Draw the ammonium salt formed in each reaction.
Draw the ammonium salt formed in each reaction (A, B,… SolvedLib
Solved Draw the ammonium salt formed in each reaction.
Solved Draw the ammonium salt formed In each reaction.
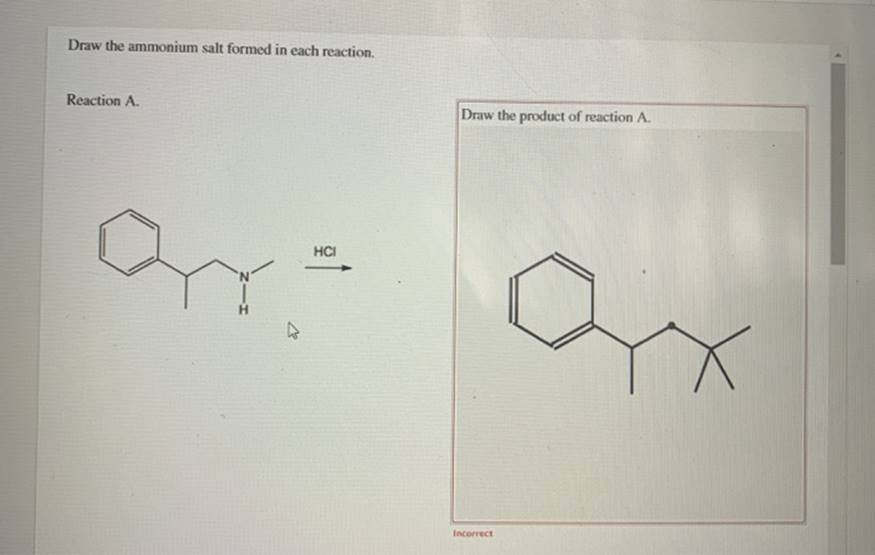
(Get Answer) Draw The Ammonium Salt Formed In Each Reaction. Reaction
Solved Draw the ammonium salt formed in each reaction.
Solved Draw the ammonium salt formed in each reaction.
Solved Draw the ammonium salt formed in each reaction.
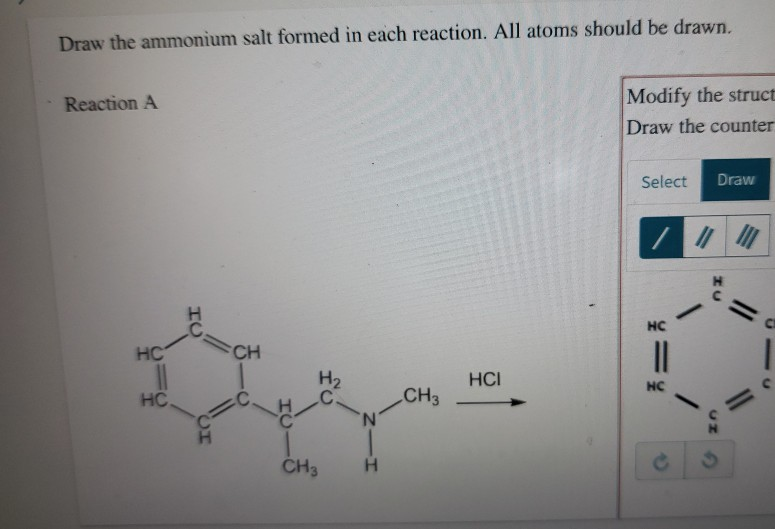
Solved Draw the ammonium salt formed in each reaction. All
Solved Draw the ammonium salt formed in each reaction.
Solved Draw the ammonium salt formed in each reaction.
Draw The Ammonium Salt Formed In Each Reaction.
We Want To Give A Balanced Reaction To The Problem.
Its Acid Ionization (Or Acid Hydrolysis) Reaction Is Represented By Nh 4 + ( A Q ) + H 2 O ( L ) ⇌ H 3 O + ( A Q ) + Nh 3 ( A Q ) K A = K W / K B Nh 4 + ( A Q ) + H 2 O ( L ) ⇌ H 3.
We Have To Draw The Ammonia Salt Formed In This Case Now.
Related Post: