Draw Resonance
Draw Resonance - When learning to draw and interpret resonance structures, there are a few basic guidelines to help. It explains how to identify the major resonance contributor as well as the minor. Web rules for drawing and working with resonance contributors. The pattern seen there is a common one that leads to a useful technique for drawing resonance forms. We need to draw another resonance structure. Want to join the conversation? 341k views 11 years ago. Web drawing resonance structures and resonance hybrid using example of nitrate anion. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Recognizing, drawing, and evaluating the relative stability of resonance contributors is essential to understanding organic reaction mechanisms. Web exceeding a critical draw ratio results in steady oscillations of both the velocity field and film or, respectively, fiber geometry, which is commonly known as draw resonance and which leads to inhomogeneous product properties and possible breakdown of the process. Why are they considered different? If it has only one lewis structure, it doesn’t have a resonance hybrid. The. Web resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Why are they considered different? We need to draw another resonance structure. Determine the relative stability of resonance structures using a set of rules. This is the acetate anion, and this dot structure does not completely describe. Want to join the conversation? How to avoid common mistakes when drawing resonance structures. Web this general chemistry video tutorial provides a basic introduction into resonance structures. Draw the lewis structure & resonance. Web draw the resonance structures of molecules or ions that exhibit delocalization. 2) do not break single bonds. There is only one π bond in this example, and no any lone pairs, so only the π electrons can be moved around. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Curved arrows and resonance structures. Web the first step of drawing resonance structures starts. The pattern seen there is a common one that leads to a useful technique for drawing resonance forms. Web drawing lewis structures: It compares and contrasts two or more possible lewis structures that can represent a particular molecule. There is a carbocation beside the π. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. How to avoid common mistakes when drawing resonance structures. Want to join the conversation? It explains how to draw the resonance structures using curved. This is the acetate anion, and this dot structure does not completely describe the acetate anion; Resonance exists only when a lewis structure has multiple bonds and an adjacent atom with at least one lone pair. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. How to identify the molecules having resonance. , aren't the three dot structures the same, just rotated? We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. We. Use the concept of resonance to explain structural features of molecules and ions. There is a carbocation beside the π. Web exceeding a critical draw ratio results in steady oscillations of both the velocity field and film or, respectively, fiber geometry, which is commonly known as draw resonance and which leads to inhomogeneous product properties and possible breakdown of the. We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. There is only one π bond in this example, and no any lone pairs, so only the π electrons can be moved around. Draw the lewis structure & resonance. Web the first step of drawing resonance structures starts with drawing. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. In both examples, the carbon ring has one atom that is bonded to two hydrogens instead of one. Recognizing, drawing, and evaluating the relative stability of resonance contributors is essential to understanding organic reaction mechanisms. We need to. If it has only one lewis structure, it doesn’t have a resonance hybrid. Keeping these in mind, go ahead and work on the following practice problems on drawing curved arrows, missing resonance forms, and determining the more stable resonance structure. Use the concept of resonance to explain structural features of molecules and ions. In both examples, the carbon ring has one atom that is bonded to two hydrogens instead of one. Web the first step of drawing resonance structures starts with drawing all the possible lewis structures. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Web exceeding a critical draw ratio results in steady oscillations of both the velocity field and film or, respectively, fiber geometry, which is commonly known as draw resonance and which leads to inhomogeneous product properties and possible breakdown of the process. It compares and contrasts two or more possible lewis structures that can represent a particular molecule. It compares and contrasts two or more possible lewis structures that can represent a particular molecule. We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. Web drawing resonance structures and resonance hybrid using example of nitrate anion. Curved arrows and resonance structures. Previously in this series on resonance, we saw that resonance forms represent two (or more) different ways to draw the same molecule, which differ only in their distribution of electrons ( see article: Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Resonance exists only when a lewis structure has multiple bonds and an adjacent atom with at least one lone pair.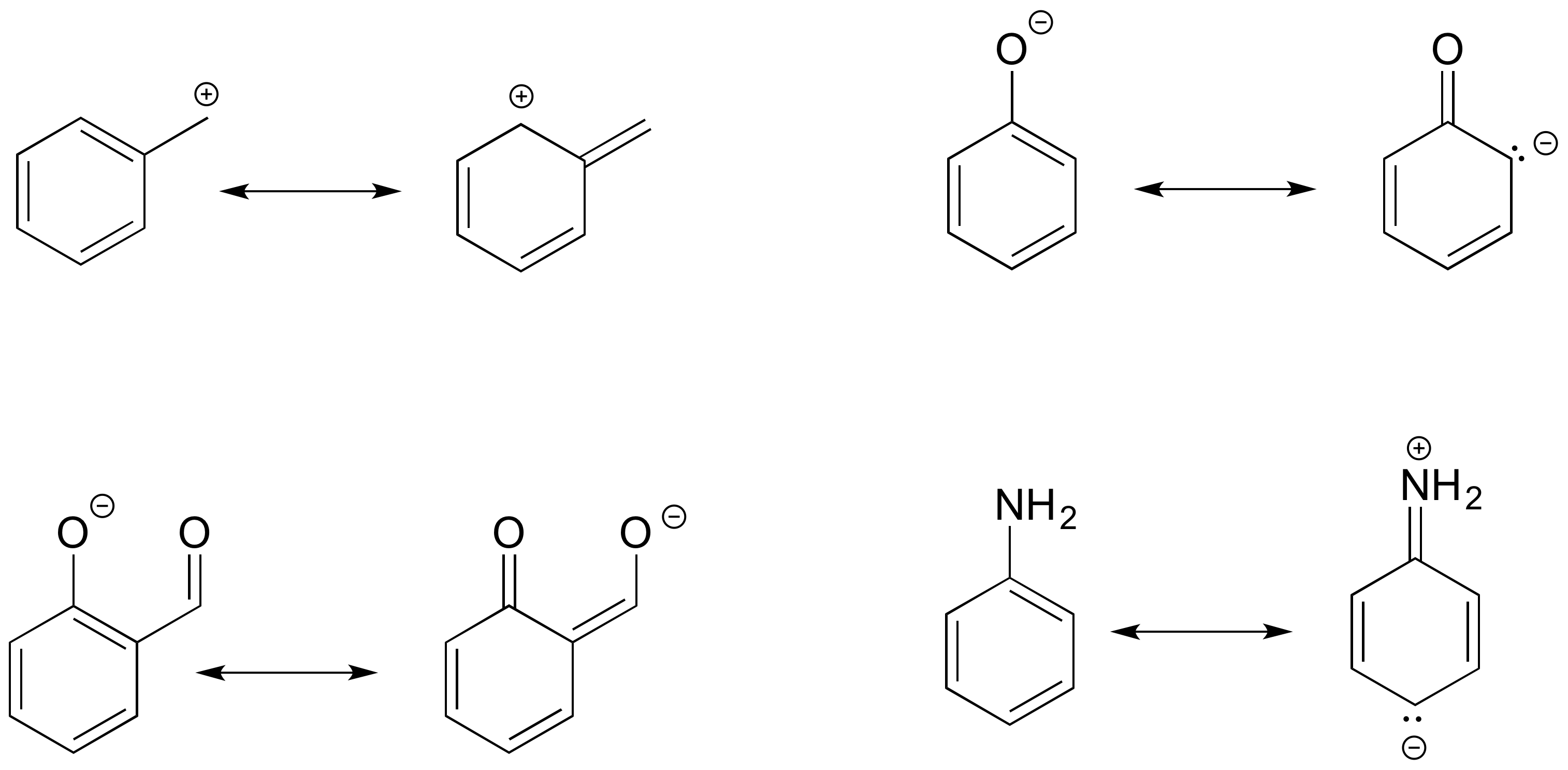
draw a second resonance structure for the following radical piano
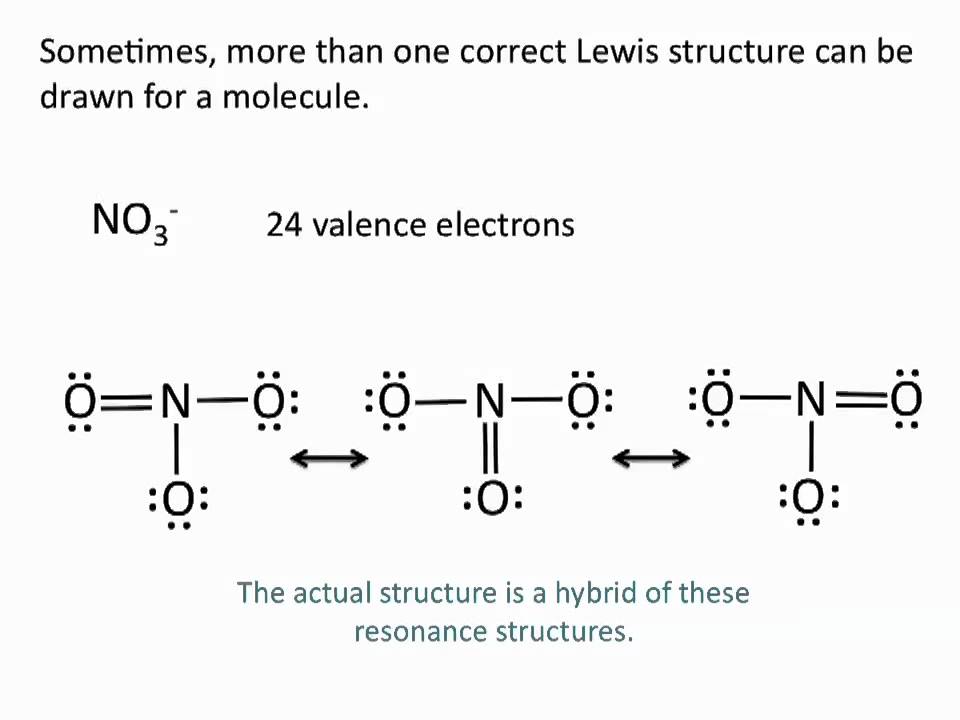
Drawing Lewis Structures Resonance Structures Chemistry Tutorial

Resonance Structures Easy Hard Science

Resonance Structures, Basic Introduction How To Draw The Resonance

Draw resonance ‒ LFMI ‐ EPFL

How to Draw Resonance Contributors MCC Organic Chemistry
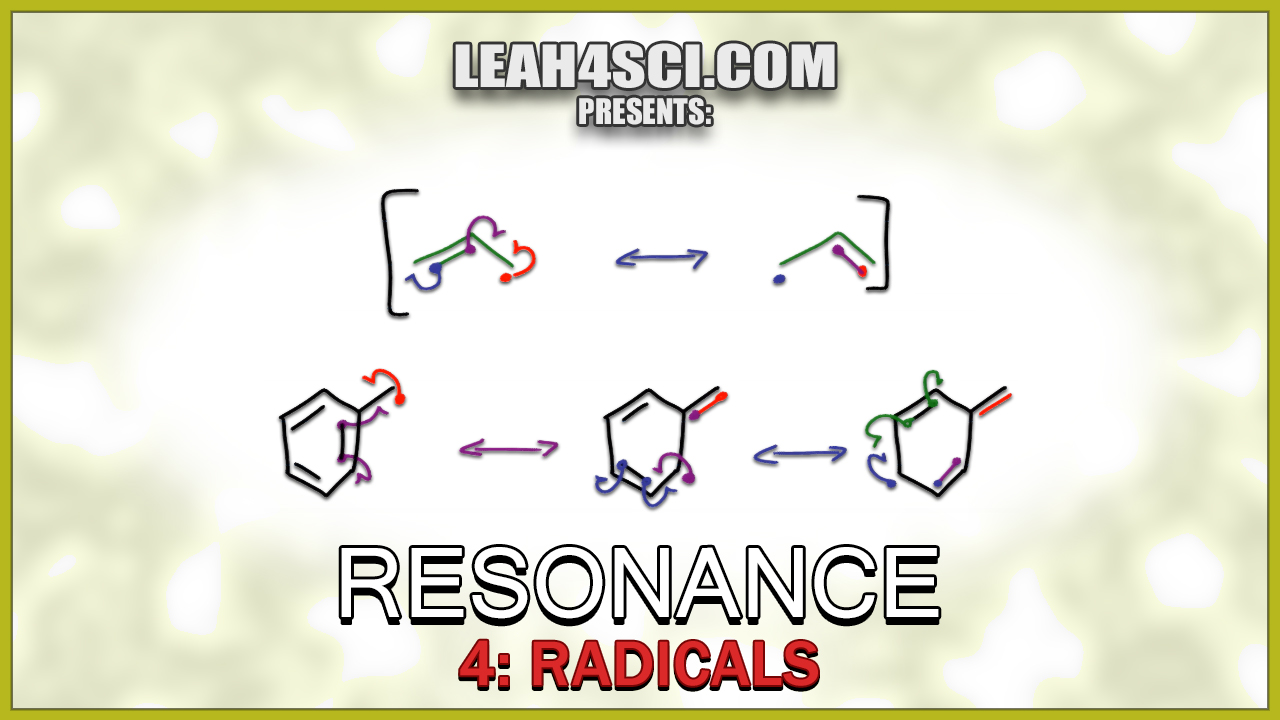
Drawing Radical Resonance for Allylic and Benzylic Radicals Tutorial Video

How to Draw Resonance Contributors MCC Organic Chemistry

Resonance Structures YouTube

How to draw resonance structures YouTube
How To Identify The Molecules Having Resonance.
When Learning To Draw And Interpret Resonance Structures, There Are A Few Basic Guidelines To Help.
Web Identify The Incorrect Resonating Structure For The Given Charged Species.
Determine The Relative Stability Of Resonance Structures Using A Set Of Rules.
Related Post: