Draw Resonance Structures For The Following Compound
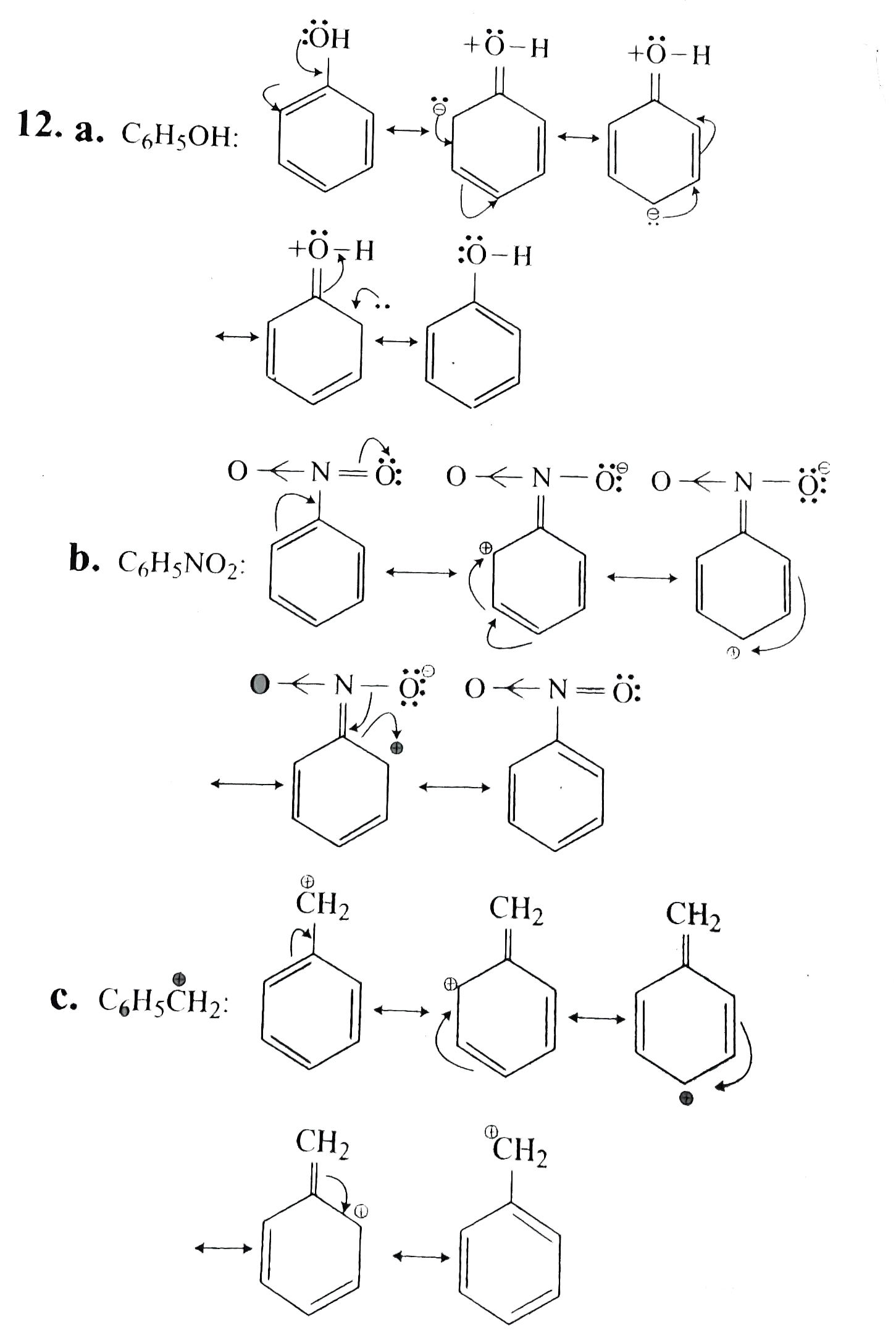
Draw Resonance Structures For The Following Compound - (iii) c h 2 = c h − c | h = o. Web draw the resonance structures of the following compounds; Web (a) the structure of c 6 h 5 oh is: Magnetic properties of complex ions. Web draw the resonance structures for the following compounds. (ii) c h 2 = c h − c h = c h 2. The resonating structures of phenol are represented as: 2.48 the significant resonance structures for the compounds (a) and (b) are drawn below. The resonating structures of nitro benzene are. When switching from general to organic chemistry, showing. (i) c h 2 = c h − c l. The resonating structures of phenol are represented as: Magnetic properties of complex ions: The resonating structures of nitro benzene are. Web there are 3 steps to solve this one. When switching from general to organic chemistry, showing. (ii) c h 2 = c h − c h = c h 2. 2.48 the significant resonance structures for the compounds (a) and (b) are drawn below. Web chemspider is a free online database of chemical structures and properties. Calculate the total number of valence electrons from each atom. (i) c h 2 = c h − c l. The resonating structures of phenol are represented as: Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. (a) `c_ (6)h_ (5)oh` (b) `c_ (6)h_ (5)no_ (2)` (. Web there are 3 steps to solve this one. Web chemspider is a free online database of chemical structures and properties. The resonating structures of phenol are represented as: Resonance is a mental exercise and method. Calculate the total number of valence electrons from each atom. Web draw the resonance structures for the following compounds. The resonating structures of nitro benzene are. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. (a) `c_ (6)h_ (5)oh` (b) `c_ (6)h_ (5)no_ (2)` (. Resonance is a mental exercise and method. (iii) c h 2 = c h − c | h = o. (a) `c_ (6)h_ (5)oh` (b) `c_ (6)h_ (5)no_ (2)` (. Web (a) the structure of c 6 h 5 oh is: The resonating structures of phenol are represented as: Resonance is a mental exercise and method. (i) c h 2 = c h − c l. You can search by structure or substructure, upload a structure file or draw using a molecule. The resonating structures of phenol are represented as: (ii) c h 2 = c h − c h = c h 2. (b) the structure of c 6 h 5 no 2 is: (i) c h 2 = c h − c l. The resonating structures of phenol are represented as: (b) the structure of c 6 h 5 no 2 is: Web (a) the structure of c 6 h 5 oh is: (ii) c h 2 = c h − c h = c h 2. Web draw the resonance structures of the following compounds; (a) `c_ (6)h_ (5)oh` (b) `c_ (6)h_ (5)no_ (2)` (. Web how to draw resonance structures. Web (a) the structure of c 6 h 5 oh is: The resonating structures of nitro benzene are. Web draw the resonance structures for the following compounds. Magnetic properties of complex ions: You can search by structure or substructure, upload a structure file or draw using a molecule. Web there are 3 steps to solve this one. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web chemspider is a free online database of chemical structures and properties. Web (a) the structure of c 6 h 5 oh is: Resonance is a mental exercise and method. Web how to draw resonance structures. Web draw the resonance structures of the following compounds; Web draw the resonance structures for the following compounds. The resonating structures of nitro benzene are. (iii) c h 2 = c h − c | h = o. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. You can search by structure or substructure, upload a structure file or draw using a molecule. Magnetic properties of complex ions. 2.48 the significant resonance structures for the compounds (a) and (b) are drawn below. Web there are 3 steps to solve this one. (i) c h 2 = c h − c l. (b) the structure of c 6 h 5 no 2 is: Resonance structures depict alternate arrangements of electrons in molecules, essential for understanding stability and reactivity ⋅. Calculate the total number of valence electrons from each atom.
Draw the resonance structures for the following compounds. Show the

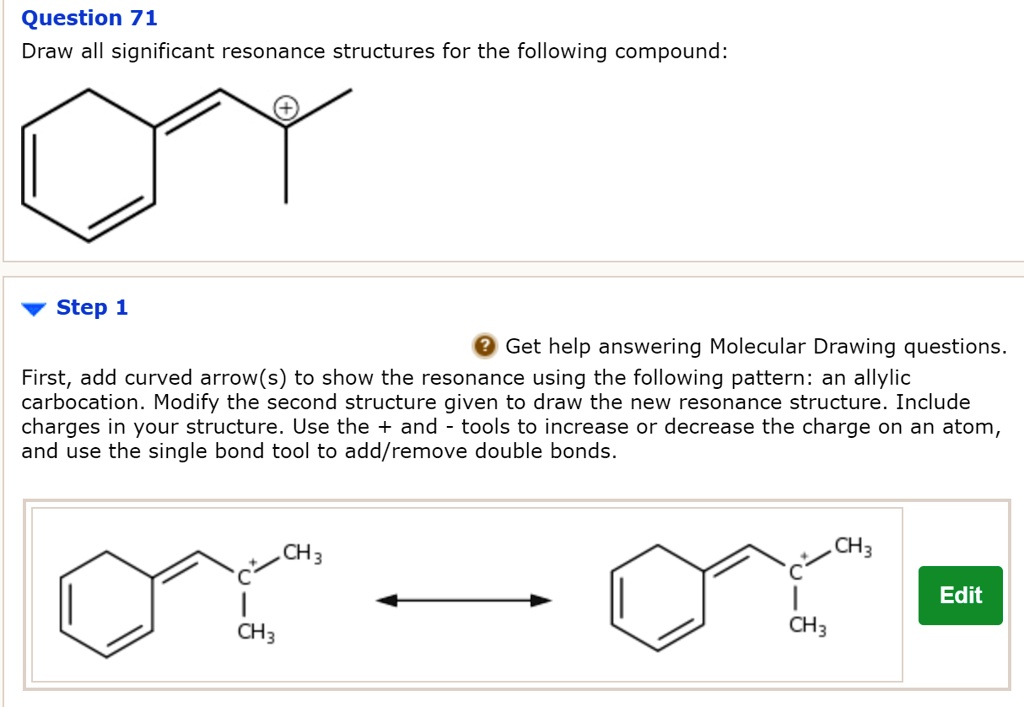
Solved Draw significant resonance structures for the
Draw the resonance structures for the following compounds. Show the el
draw significant resonance structures for the following compound
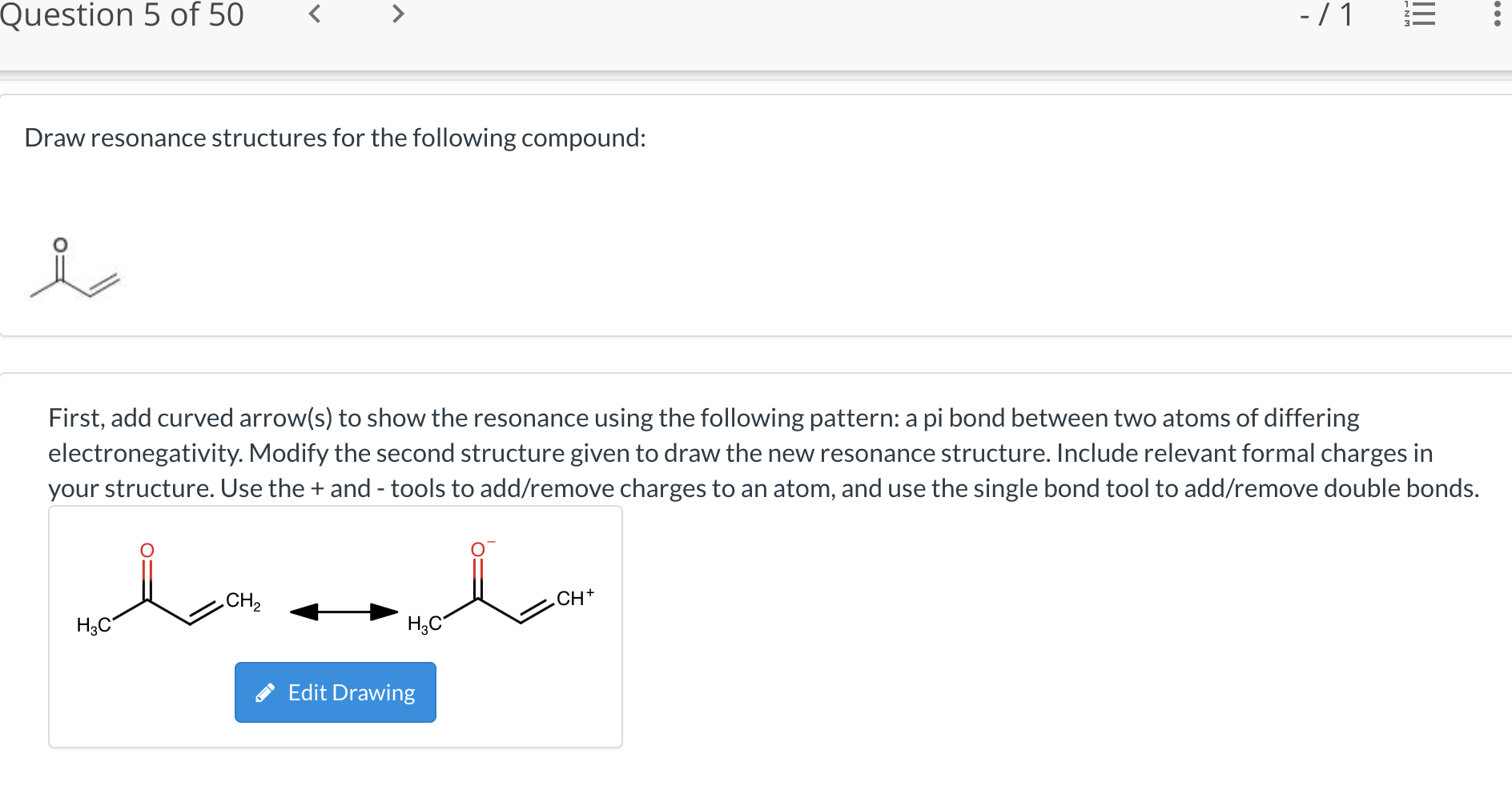
Solved Draw resonance structures for the following compound
Solved Draw resonance structures for the following compound

Draw a Resonance Structure for the Compound Below.
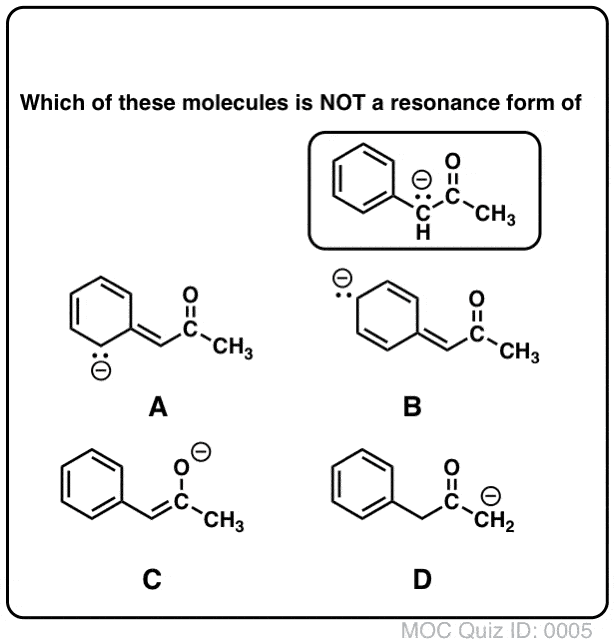
Draw Significant Resonance Structures for the Following Compound

1.3 Resonance Structures Organic Chemistry I
[Solved] Draw the resonance structures for each compound
When Switching From General To Organic Chemistry, Showing.
Magnetic Properties Of Complex Ions:
(A) `C_ (6)H_ (5)Oh` (B) `C_ (6)H_ (5)No_ (2)` (.
The Resonating Structures Of Phenol Are Represented As:
Related Post:
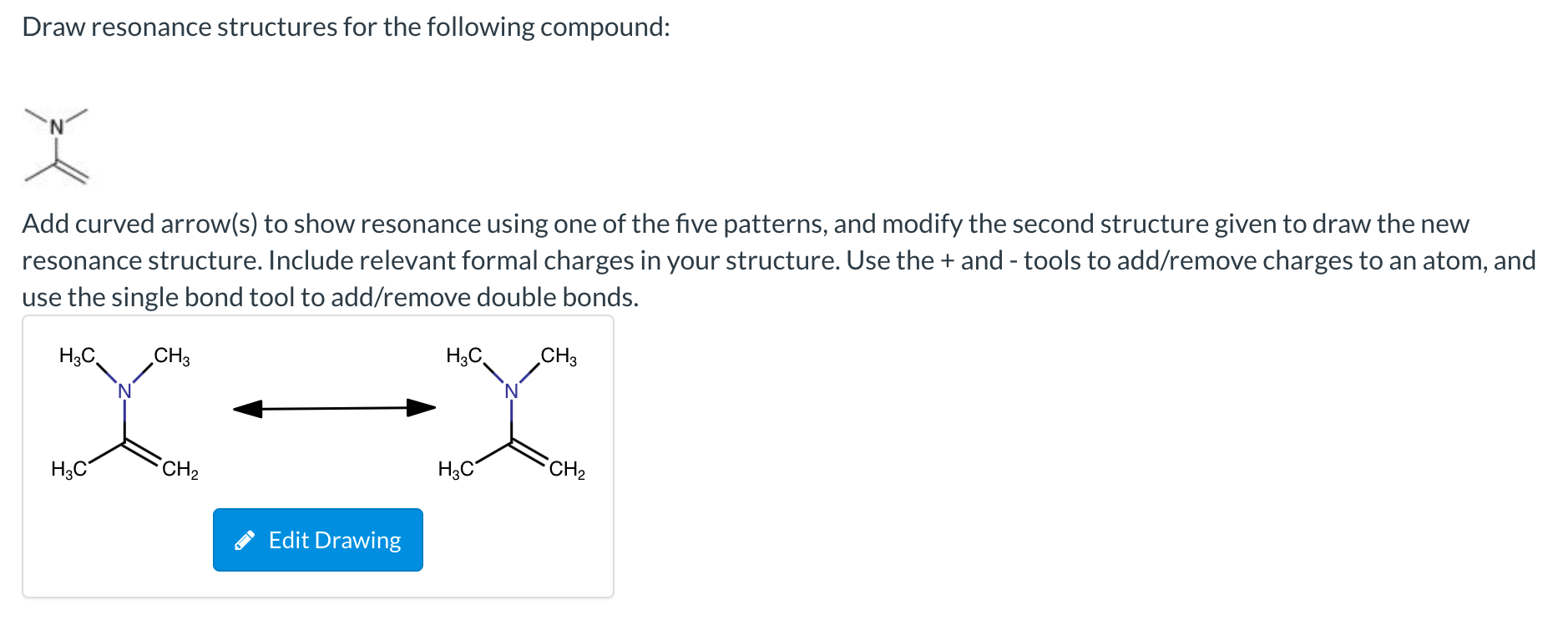
![[Solved] Draw the resonance structures for each compound](https://media.cheggcdn.com/study/610/61043755-bf9b-45e2-a076-c7cceb4aa755/image)