Draw Lewis Structure For So2
Draw Lewis Structure For So2 - Calculate the total number of valence electrons. Here, a = central atom, x = surrounding atoms and e = the lone pairs. 146k views 3 years ago. Web home » basic chemistry. A lewis structure is a way to show how atoms share electrons when they form a molecule. January 15, 2024 by jyoti bashyal. There are two oxygen atoms bonded to the central sulfur atom. The formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with two double bonds. Web home | science | chemistry. What is the hybridization of sulfur in the lewis structure of so2? Web in so2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. I have seen two different ways the lewis structure is written: Note that so2 is a. The formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with two double bonds. (valence electrons are the number of electrons present in the outermost shell of an atom). Decide which is the central atom in the structure. You might think you've got the correct lewis structure for so. Remember, sulfur is in period 3 and can hold more than 8 valence electrons. In so2, sulfur is in group 6, so it has 6 valence electrons, while each oxygen atom in group 6 contributes 6 valence electrons. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Determine the total valence electrons. First. This problem has been solved! First u have to draw all electrons around s (6 electrons) and around two o (each 6 electrons). 14k views 11 years ago chemistry lewis dot structures. A lewis structure is a way to show how atoms share electrons when they form a molecule. Determine the total valence electrons. 534k views 10 years ago. Here, a = central atom, x = surrounding atoms and e = the lone pairs. Lewis structures show all of the valence electrons in an atom or molecule. Web the dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that. Decide which is the central atom in the structure. 14k views 11 years ago chemistry lewis dot structures. So2 lewis structure shows various chemical properties of the compound. Web there are three resonance structures so2 (sulfur dioxide). There is also a lone pair attached to the sulfur atom. Web in so2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. (valence electrons are the number of electrons present in the outermost shell of an atom). Calculate the total number of valence electrons. Drawing the lewis structure of so2 is critical for understanding its molecular bonding and chemical characteristics. A lewis structure is a way. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Then you have to know that oxygen loves to make two covalent bonds with another atom/s. 146k views 3 years ago. View the full answer answer. Web to draw the so2 lewis structure, follow these simple steps: Calculate the total number of valence electrons. Do not consider ringed structures. Note that so2 is a bit. Web home » basic chemistry. Draw the best lewis structure for so2 by filling in the bonds, lone pairs, and formal charges. What is the molecular geometry of so2 based on its lewis structure? Sulfur ( s) is in group 16 of the periodic tab. So2 lewis structure shows various chemical properties of the compound. Count the total number of valence electrons: Draw a trial structure by putting electron pairs around every atom until each gets an octet. Drawings, hybridization, shape, charges, pair and detailed facts. This chemistry video tutorial explains how to draw the lewis structure of so2 also known as sulfur dioxide. Count the total number of valence electrons: Web in so2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. 534k views 10 years ago. S and o atoms have sp2 hybridization. Web to draw the so2 lewis structure, follow these simple steps: Determine the total valence electrons. Here is a little flow chart of how we are going to do this: In so2, sulfur is in group 6, so it has 6 valence electrons, while each oxygen atom in group 6 contributes 6 valence electrons. May 25, 2022 by upasana nayak. How many valence electrons does sulfur dioxide (so2) have? The formal charges of the so 2 with the single bond and a double bond is larger than the so 2 with two double bonds. Web how do you draw the lewis structure for sulfur dioxide (so2)? Sulfur dioxide lewis structure is drawn step by step using vespr rules. There are two oxygen atoms bonded to the central sulfur atom.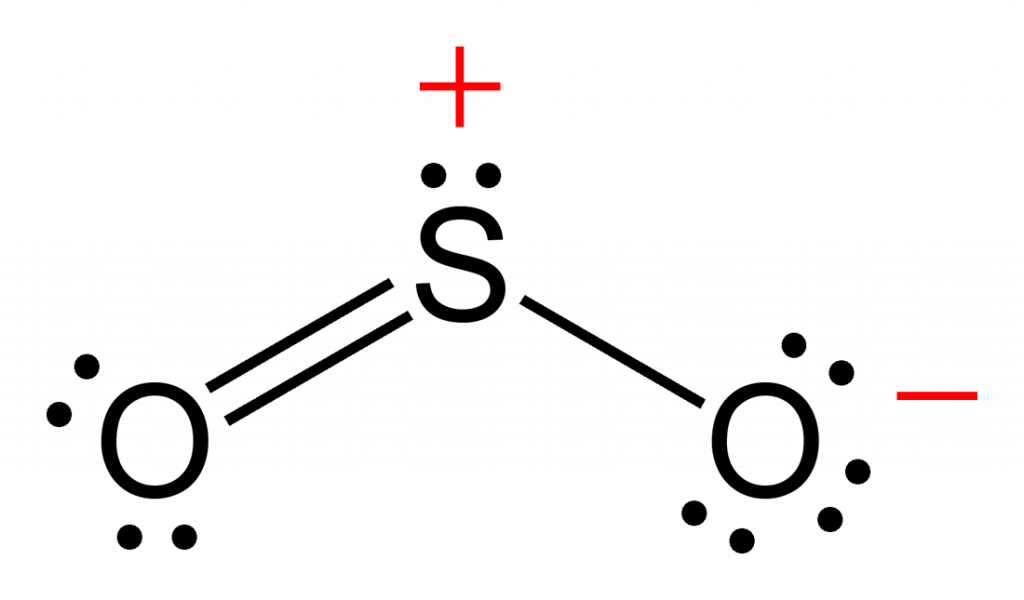
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of

How to draw SO2 Lewis Structure? Science Education and Tutorials
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
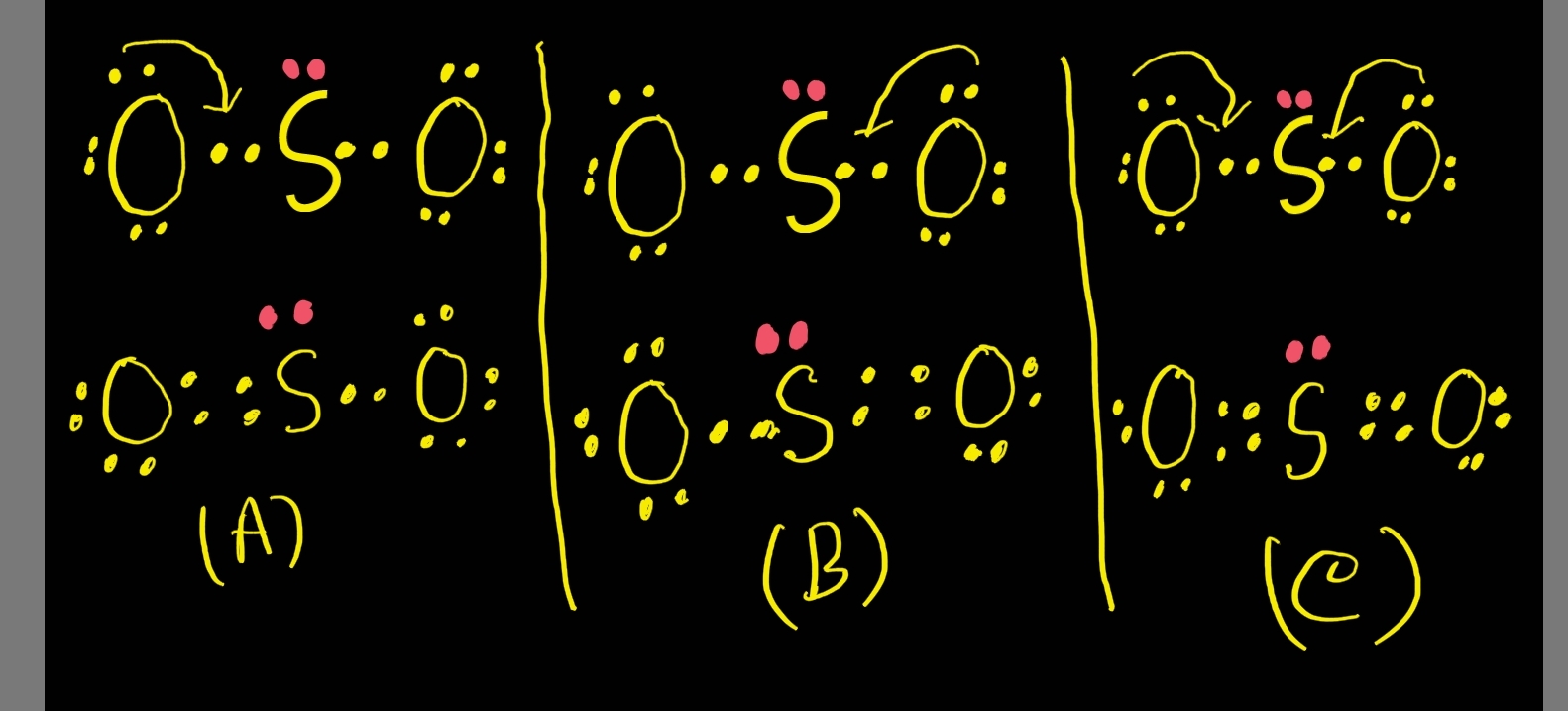
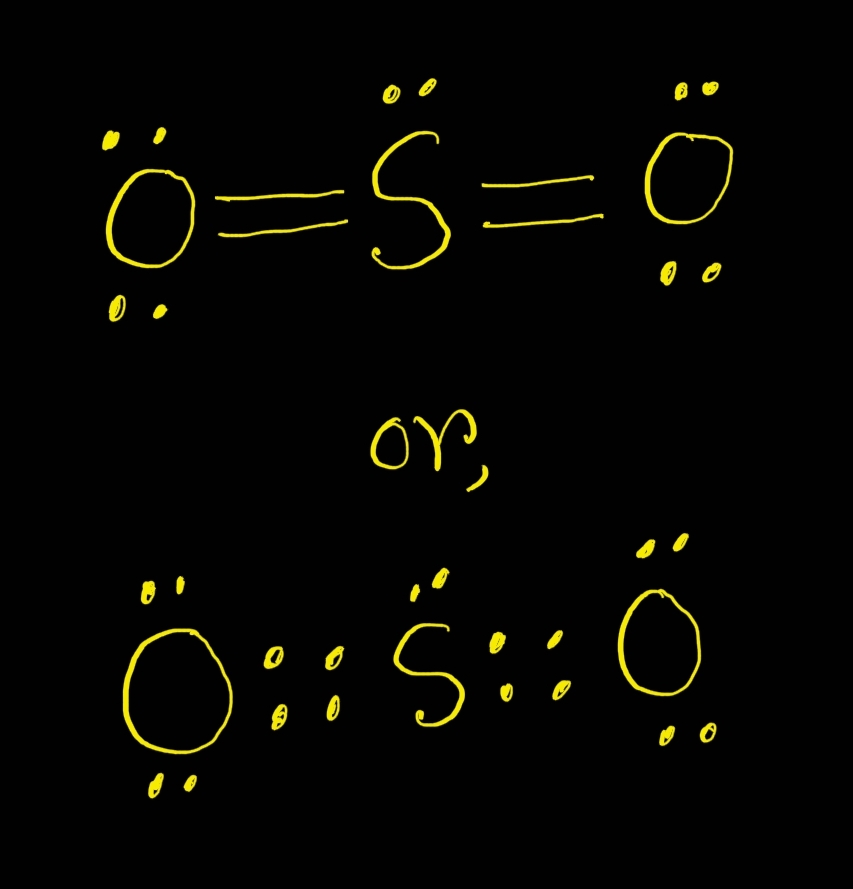
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar

So2 Lewis Structure Resonance images
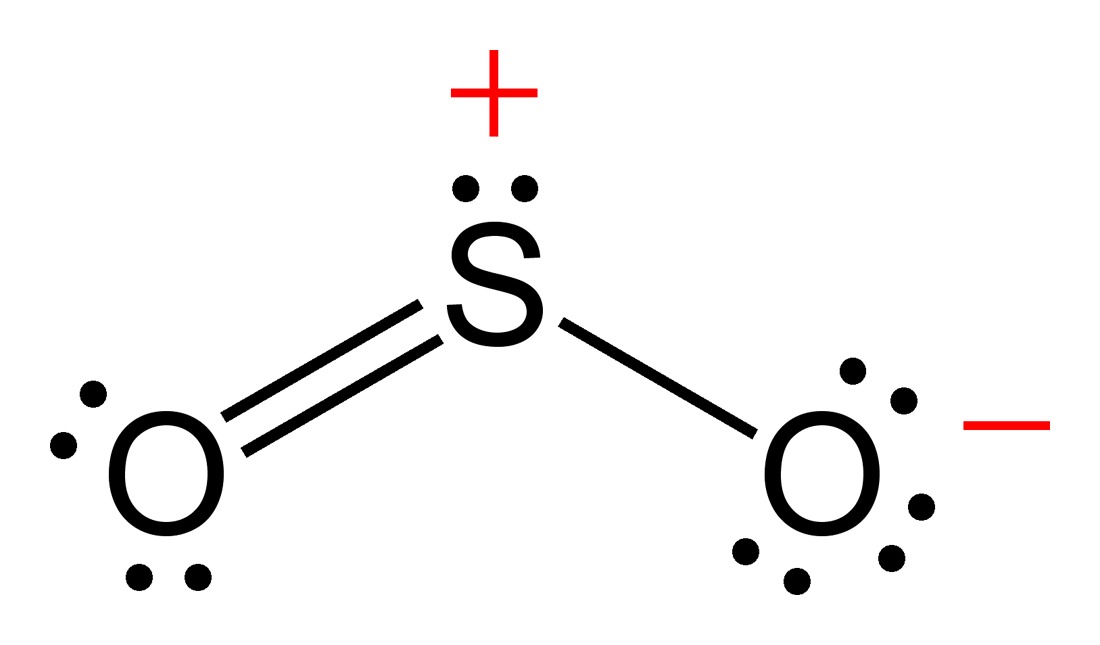
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of

How to draw SO2 Lewis Structure? Science Education and Tutorials
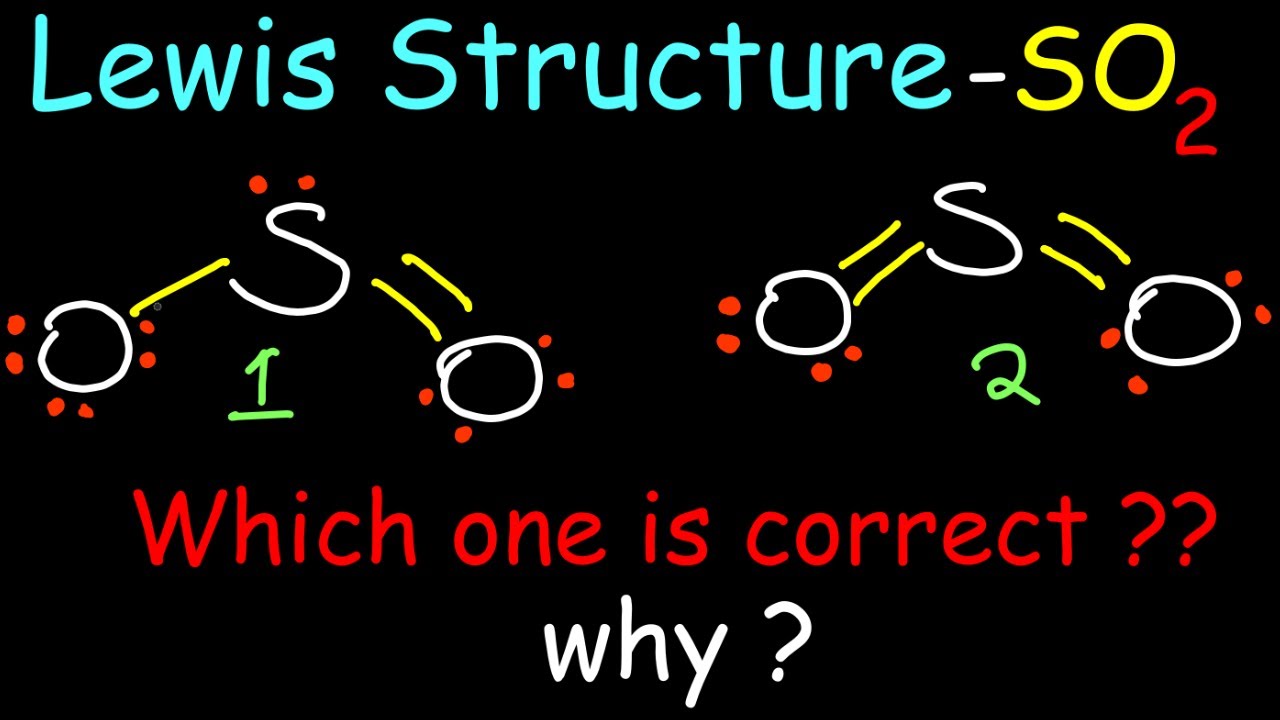
Lewis Structure of Sulphur Dioxide SO2 YouTube

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
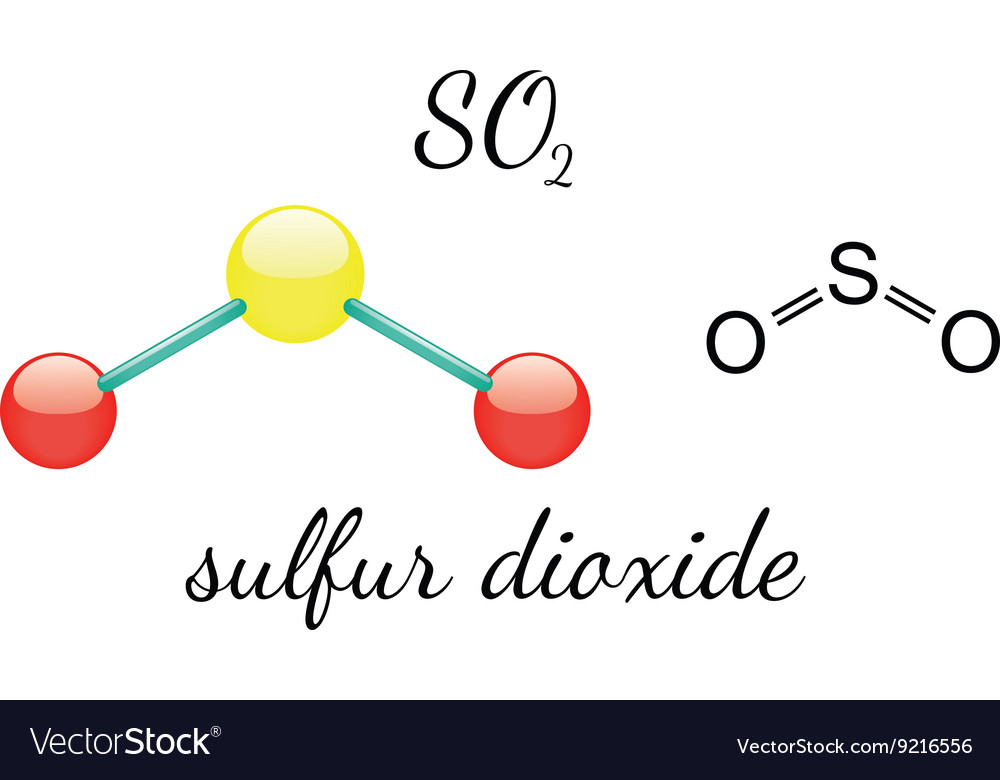
So2sulfur Dioxide Molecular Geometry Lewis Structure
Drawing The Lewis Structure Of So2 Is Critical For Understanding Its Molecular Bonding And Chemical Characteristics.
We Start With A Valid Lewis Structure And Then Follow These General Rules.
So I Would Assume That The One With Two Double Bonds Is The Correct Structure.
There Is Also A Lone Pair Attached To The Sulfur Atom.
Related Post: