Draw H2O
Draw H2O - Web here, we need to understand how the lewis structure is drawn for the h2o molecule: Construct salcs and the molecular orbital diagram for h 2 o. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. O has 6 valence electrons, and each h has one. 84k views 11 years ago. It is four for one water (h2o) molecule. A lewis structure is a way to show how atoms share electrons when they form a molecule. It is eight to form a single h2o molecule. Here, the given molecule is h2o (water). Identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. 162k views 12 years ago every video. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Web try to draw the h 2 o lewis structure before. First, we need to draw the lewis structure of h 2 o. A lewis structure is a way to show how atoms share electrons when they form a molecule. Web follow these steps to draw the lewis structure of h2o: Count the total valence electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent (. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. It is eight to form a single h2o molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Here, the given molecule is h2o (water). O has 6 valence electrons, and each h. Web 6 steps to draw the lewis structure of h2o. You must arrange 8 electrons in pairs so that. You can find a procedure for drawing lewis structures at this location. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. In short, these are the steps you. As heat is added, the temperature of the ice increases linearly with time. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. 162k views 12 years ago every video. To draw lewis structures for molecules and polyatomic ions with one central atom. O has 6 valence electrons, and each h. As heat is added, the temperature of the ice increases linearly with time. A lewis structure is a way to show how atoms share electrons when they form a molecule. Construct salcs and the molecular orbital diagram for h 2 o. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. I also go over hybridization, shape. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. For h₂o, o must be the central atom. Web follow these. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). 162k views 12 years ago every video. I also go over hybridization, shape. Web a water molecule consists of two. Count the total valence electrons. In short, these are the steps you need to follow for drawing a lewis structure: As heat is added, the temperature of the ice increases linearly with time. 162k views 12 years ago every video. Web 6 steps to draw the lewis structure of h2o. Water (h2o) geometry and hybridization. Calculate the total number of valence electrons. 162k views 12 years ago every video. In short, these are the steps you need to follow for drawing a lewis structure: It is eight to form a single h2o molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Web try to draw the h 2 o lewis structure before watching the video. Look for how many electrons are needed: 84k views 11 years ago. Water (h2o) geometry and hybridization. A lewis structure is a way to show how atoms share electrons when they form a molecule. Here, the given molecule is h2o (water). This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. To draw lewis structures for molecules and polyatomic ions with one central atom. Write the correct skeletal structure for the molecule. It is eight to form a single h2o molecule. The sample is initially ice at 1 atm and −23°c; For the h2o structure use the periodic table to find the total number of valence electrons for the h2o. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. I also go over hybridization, shape. Determine the total number of valence electrons for all the atoms in the molecule.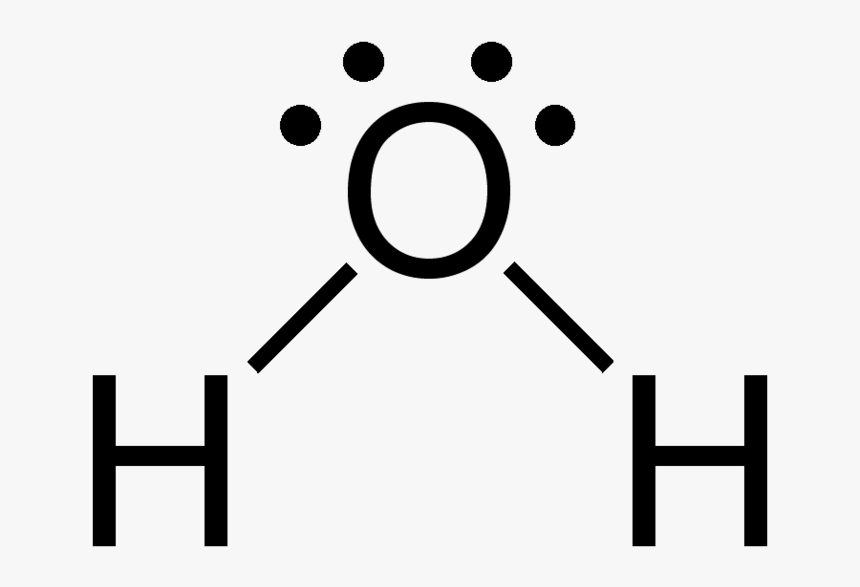
Draw Step By Step The Lewis Structure For Water (H2O)

H2O Lewis Structure, Molecular Geometry, and Hybridization
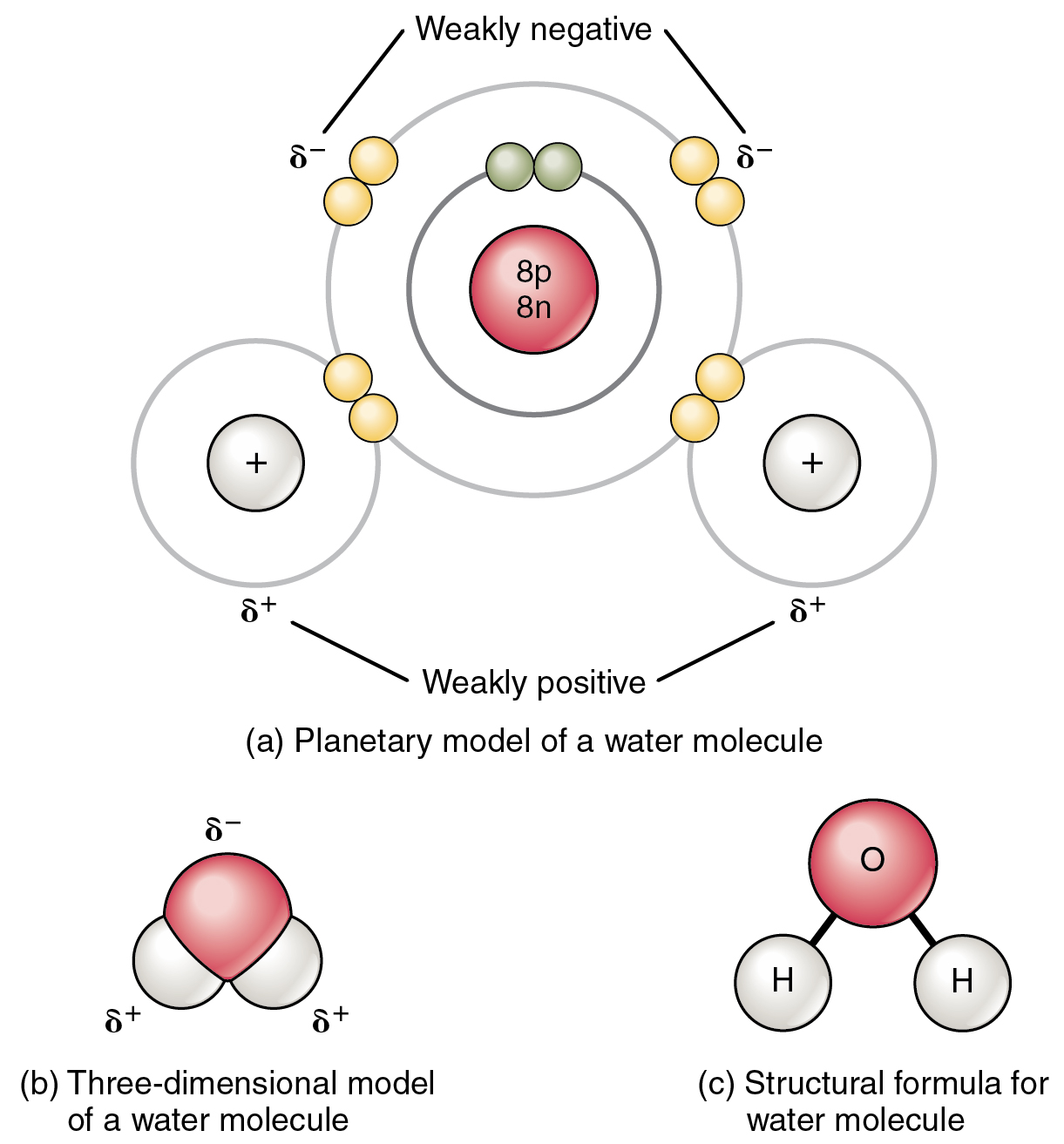
Chemical Bonds · Anatomy and Physiology

Draw The Lewis Structure Of H2O

Draw Step By Step The Lewis Structure For Water (H2O)

H2O Lewis Structure, Molecular Geometry, and Hybridization

H2o water molecule Royalty Free Vector Image VectorStock
Chemistry model of molecule water H2O scientific elements. Integrated
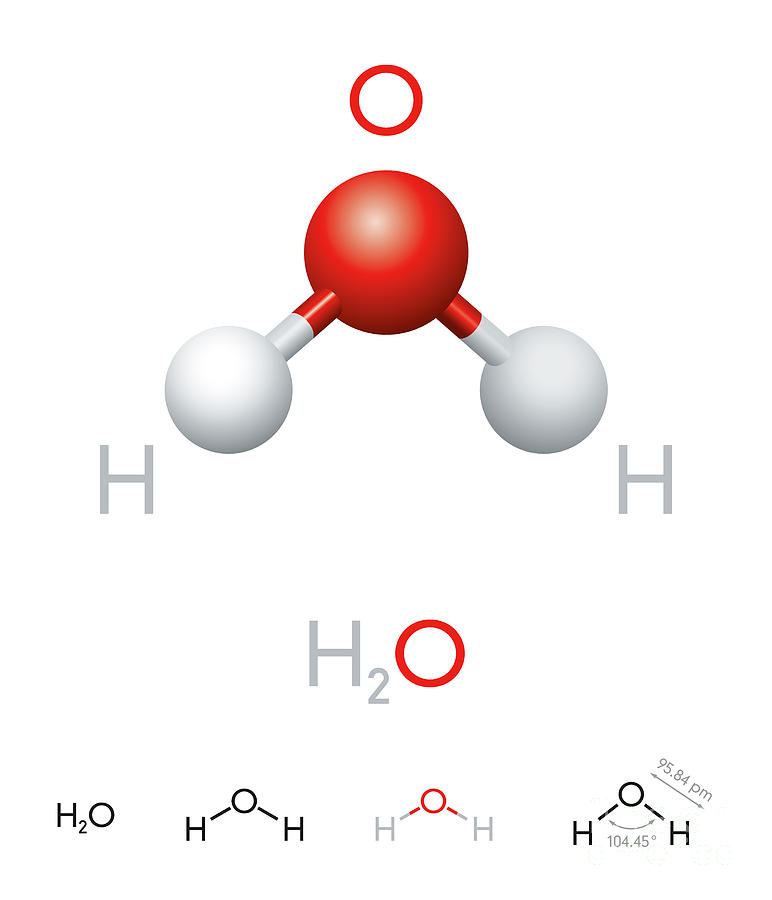
H2O Water molecule model and chemical formula Digital Art by Peter

How to Draw Water Step by Step For Kids & Beginners
First, We Need To Draw The Lewis Structure Of H 2 O.
Find The Point Group Of The Molecule And Assign Cartesian Coordinates So That Z Is The Principal Axis.
Watch The Video And See If You Missed Any Steps Or Information.
Try Structures Similar To H 2 O (Like Hcl Or Ch 4) For More Practice.
Related Post: