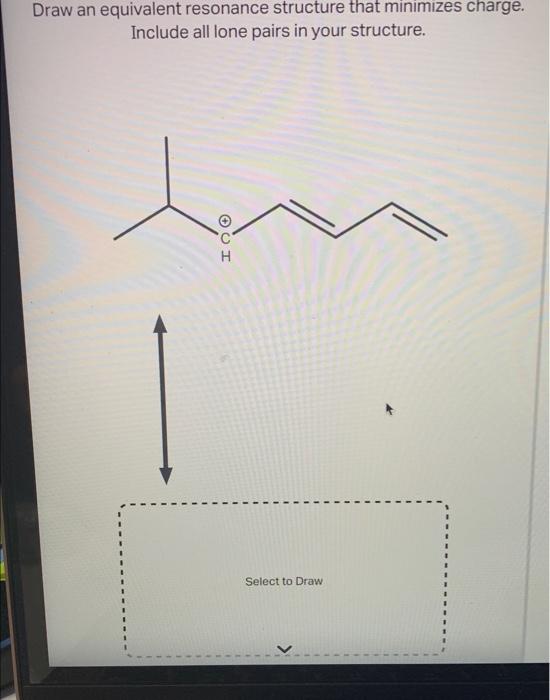
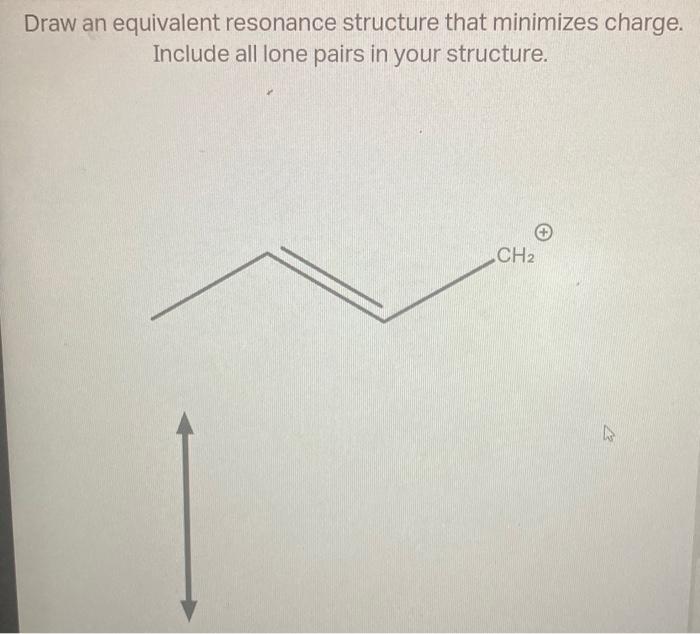
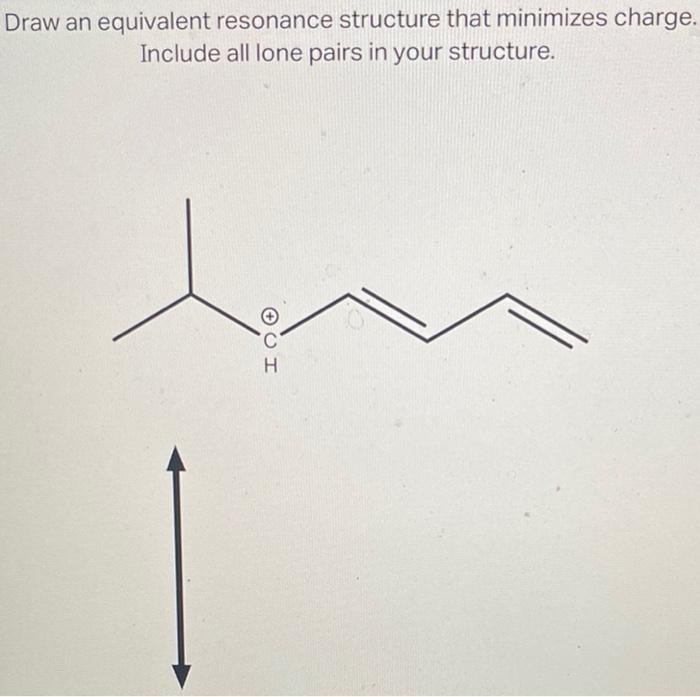
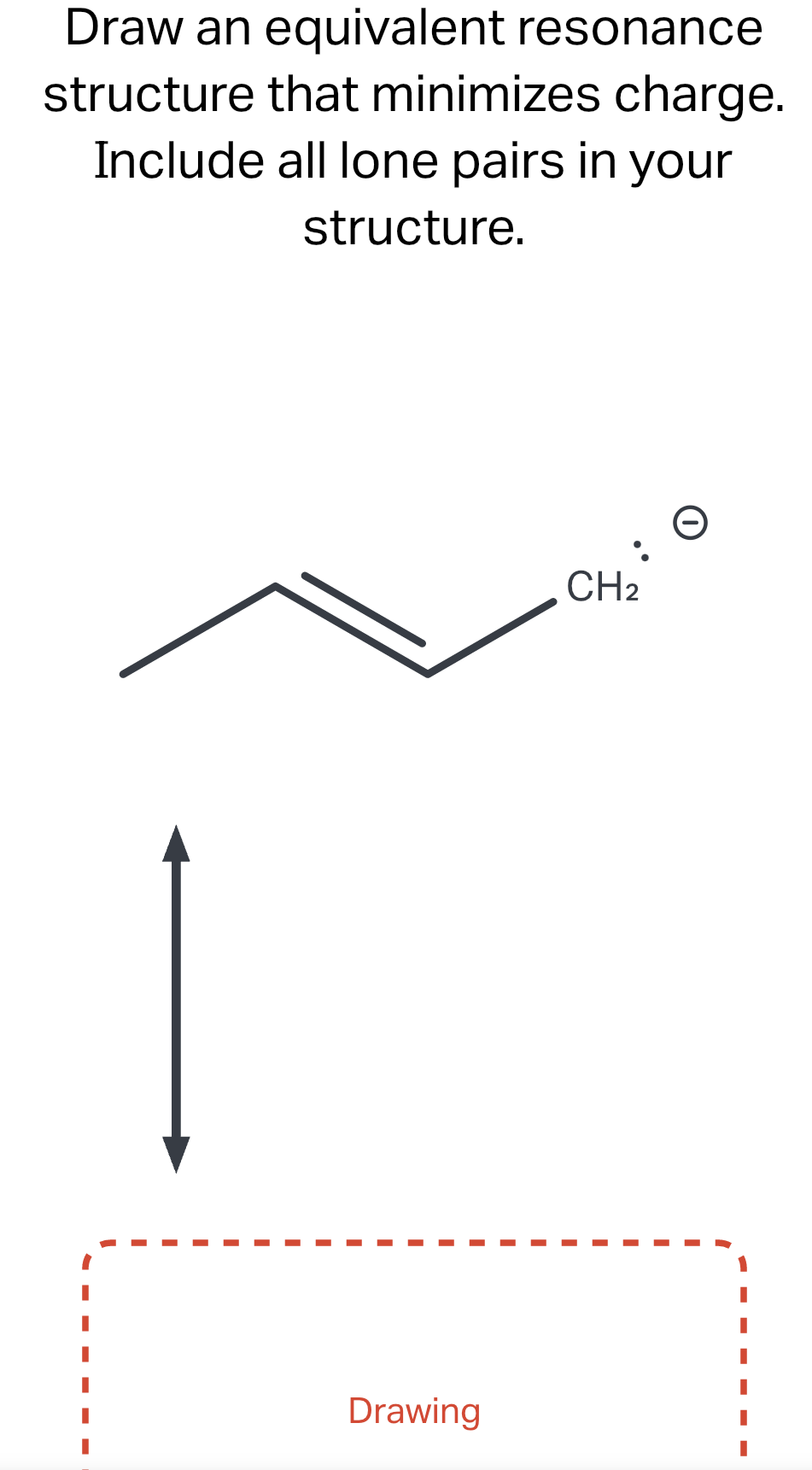
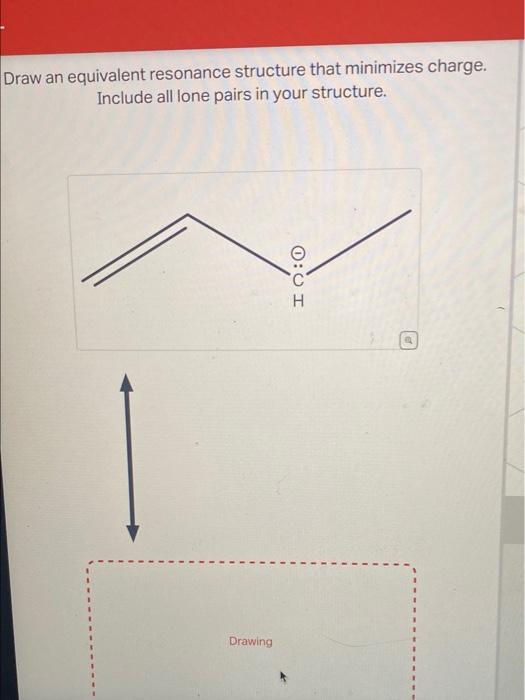
Draw An Equivalent Resonance Structure That Minimizes Charge
Draw An Equivalent Resonance Structure That Minimizes Charge - Web draw an equivalent resonance structure that minimizes charge. This is important because neither resonance structure. Evaluating the resonance structures of ethene (“ethylene”) evaluating the. Web draw an equivalent resonance structure that minimizes charge. Web the first rule of evaluating resonance structures: Web use formal charges to identify the most reasonable lewis structure for a given molecule. Web draw resonance structures and evaluate their relative contributions to the resonance hybrid using formal charges and the octet rule. There are some cases in which more than one viable lewis structure can be drawn for a molecule. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. | bond order, bond length, and bond. There are some cases in which more than one viable lewis structure can be drawn for a molecule. | bond order, bond length, and bond. Draw an equivalent resonance structure that minimizes charge. Include all lone pairs in your structure. Draw an equivalent resonance structure that minimizes charge. Web draw an equivalent resonance structure that minimizes charge. Use formal charges to identify the most reasonable lewis structure for a given molecule; Draw or tap a new bond to. Web while both resonance structures are chemically identical, the negative charge is on a different oxygen in each. Web draw an equivalent resonance structure that minimizes charge. Web draw the resonance structures of molecules or ions that exhibit delocalization. Identify the type of organic compound shown below: Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. | bond order, bond length, and bond. Include all lone pairs in your structure. Web it’s a valid resonance structure but it’s not a good one because it now has 3 formal charges instead of 1. Identify the type of organic compound shown below: Web draw an equivalent resonance structure that minimizes charge.include all lone pairs in your structure.probledraw an equivalent resonance structure that minimizes. Include all lone pairs in your structure. Determine the. Include all lone pairs in your structure. Include all lone pairs and charges as appropriate. Include all lone pairs in your structure. Additionally it has a positive charge directly next to a negative. Web draw an equivalent resonance structure that minimizes charge. Draw an equivalent resonance structure that minimizes charge. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web in terms of formal charge, a structure generally contributes more when (1) the formal charges on the atoms are minimized and (2) any negative formal charges are on more. This is important because neither resonance structure. Web. Include all lone pairs in your structure. Web while both resonance structures are chemically identical, the negative charge is on a different oxygen in each. Resonance is a mental exercise and. Web draw all possible resonance structures for each of the compounds below. Evaluating the resonance structures of ethene (“ethylene”) evaluating the. Include all lone pairs in your structure. Consider ozone (o 3) solution. Use formal charges to identify the most reasonable lewis structure for a given molecule; Web compute formal charges for atoms in any lewis structure; Draw an equivalent resonance structure that minimizes charge. Web draw the resonance structures of molecules or ions that exhibit delocalization. This is important because neither resonance structure. Web the first rule of evaluating resonance structures: Web in terms of formal charge, a structure generally contributes more when (1) the formal charges on the atoms are minimized and (2) any negative formal charges are on more. Include all lone. Include all lone pairs in your structure. Web draw an equivalent resonance structure that minimizes charge.include all lone pairs in your structure.probledraw an equivalent resonance structure that minimizes. An example is the ozone \(\left( \ce{o_3} \right)\). Web use formal charges to identify the most reasonable lewis structure for a given molecule. | bond order, bond length, and bond. Include all lone pairs in your structure. Include all lone pairs in your structure. Web while both resonance structures are chemically identical, the negative charge is on a different oxygen in each. Web the first rule of evaluating resonance structures: Additionally it has a positive charge directly next to a negative. Determine the relative stability of resonance structures using a set of rules. Web draw an equivalent resonance structure that minimizes charge.include all lone pairs in your structure.probledraw an equivalent resonance structure that minimizes. Determine the formal charge on each atom in each of the resonance structures: Web draw an equivalent resonance structure that minimizes charge. | bond order, bond length, and bond. Identify the type of organic compound shown below: Web in terms of formal charge, a structure generally contributes more when (1) the formal charges on the atoms are minimized and (2) any negative formal charges are on more. Include all lone pairs in your structure. Web draw the resonance structures of molecules or ions that exhibit delocalization. There are some cases in which more than one viable lewis structure can be drawn for a molecule. Include all lone pairs in your structure.[Solved] Draw an equivalent resonance structure that minimizes charge
[Solved] . Draw an equivalent resonance structure that minimizes charge
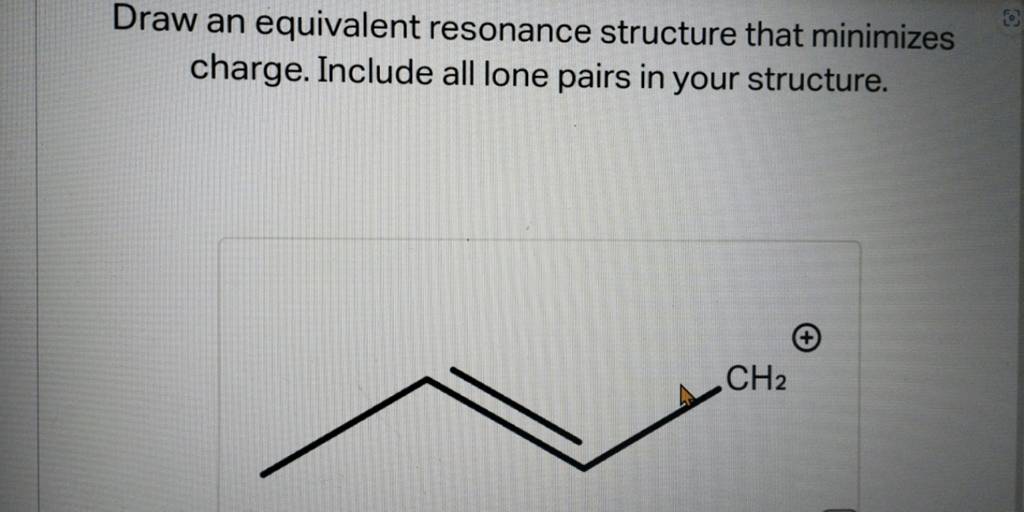
Draw an equivalent resonance structure that minimizes charge. Include all..
Solved Draw an equivalent resonance structure that minimizes
[Solved] Draw an equivalent resonance structure that minimizes charge
Solved Draw an equivalent resonance structure that minimizes
Solved Draw an equivalent resonance structure that minimizes
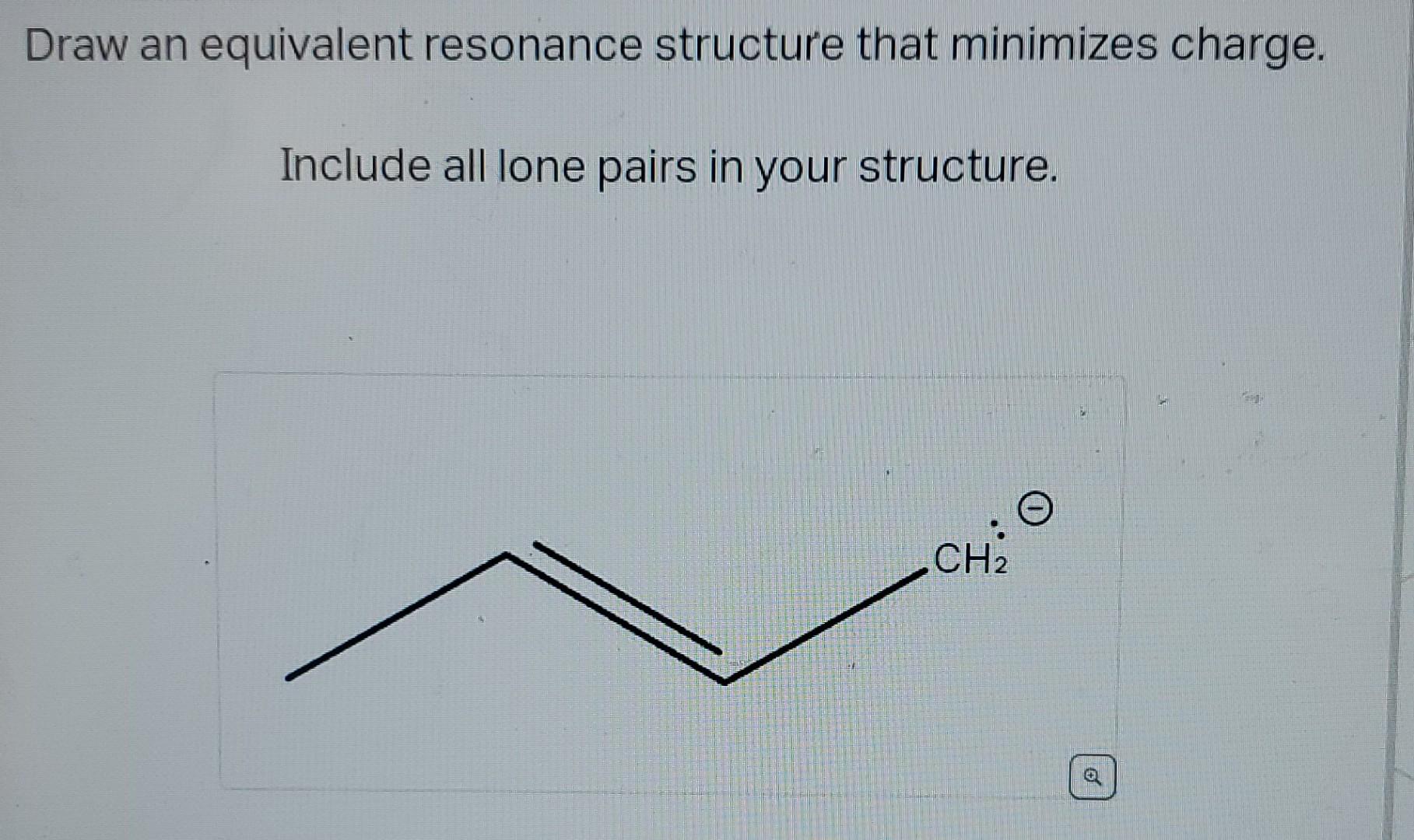
Solved Draw an equivalent resonance structure that minimizes
Solved Draw an equivalent resonance structure that minimizes
Solved Draw an equivalent resonance structure that minimizes
Web Draw Resonance Structures And Evaluate Their Relative Contributions To The Resonance Hybrid Using Formal Charges And The Octet Rule.
An Example Is The Ozone \(\Left( \Ce{O_3} \Right)\).
Include All Lone Pairs In Your Structure.
Some Molecules Have Two Or More Chemically Equivalent Lewis Electron Structures, Called Resonance Structures.
Related Post: