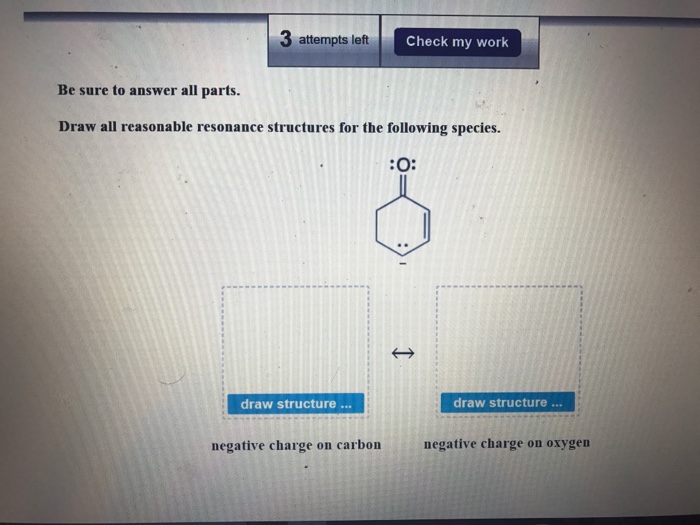
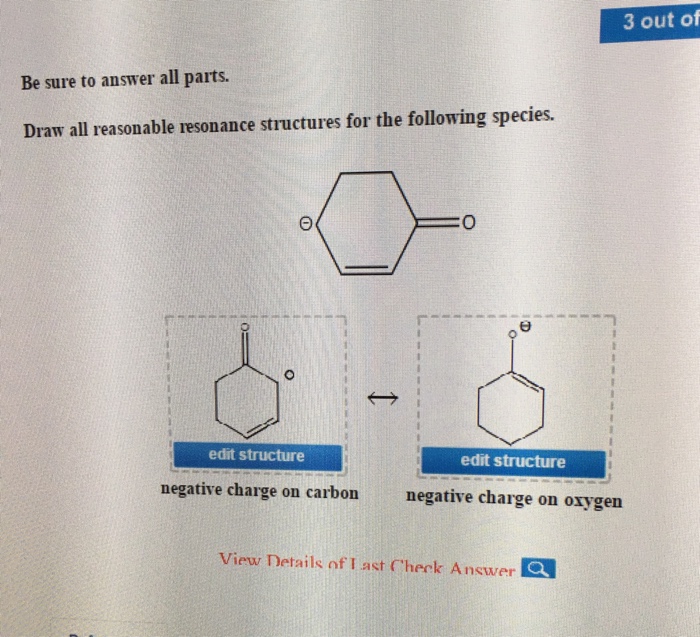
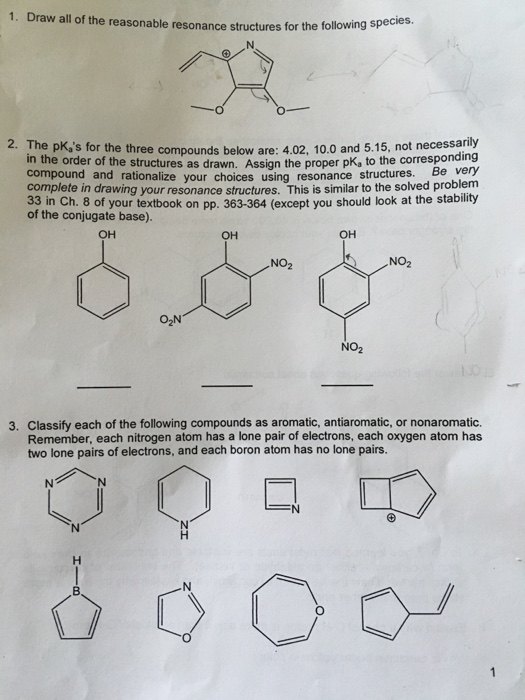
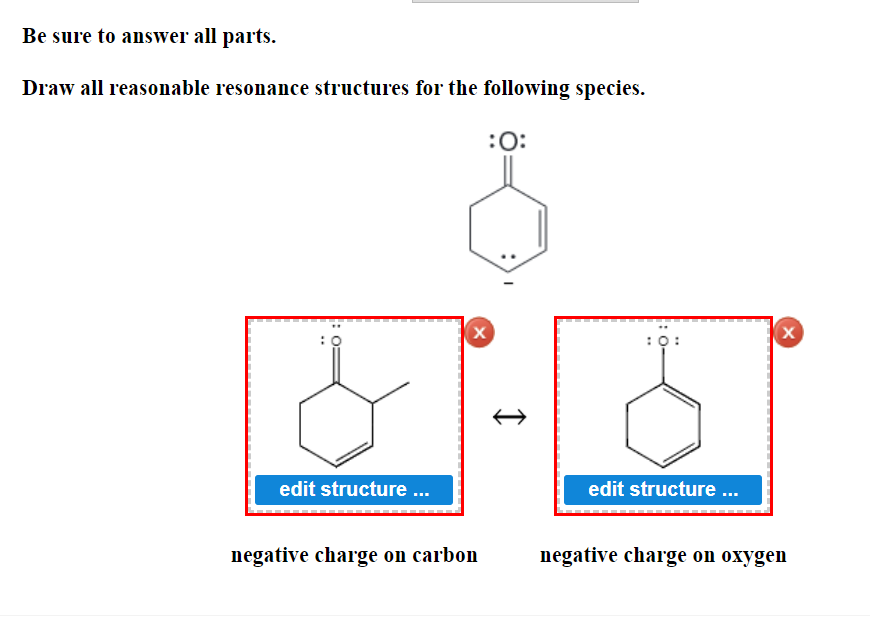
Draw All Reasonable Resonance Structures For The Following Species
Draw All Reasonable Resonance Structures For The Following Species - Draw resonance structures for the following species. Determine the relative stability of resonance structures using a set of. We always try to draw resonance that has the minimum possible change. Choose the most favorable lewis structure. Draw all reasonable resonance structures for the following species. For o 3, each oxygen atom contributes 6 valence electrons, so the total is 3 × 6 = 18 electrons. Determine the location of the double bonds or lone pairs in the molecule. For the rat, generate a resonance structure that includes the following. Draw all of the reasonable resonance structures for each of the following molecules. (24 pts) draw all reasonable resonance structures for the following species. For the human, generate a resonance structure that includes the following elements: Draw resonance structures for the following species. The molecule can represent a. Identify the species for which we need to draw resonance structures. Use arrows to show the apparent motion of electron. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web be sure to answer all parts. Draw all reasonable resonance structures for the given species. Draw all reasonable resonance structures for the following species. For o 3, each oxygen atom contributes 6 valence electrons, so the total is 3 × 6 = 18 electrons. First, we need to determine the total number of valence electrons for each species. We always try to draw resonance that has the minimum possible change. Draw resonance structures for the following species. Web draw the resonance structures of molecules or ions that exhibit delocalization. The molecule can represent a. A, g, c, and t. (24 pts) draw all reasonable resonance structures for the following species. For the rat, generate a resonance structure that includes the following. Negative charge on carbon negative. Use arrows to show the apparent motion of electron. We always try to draw resonance that has the minimum possible change. For the human, generate a resonance structure that includes the following elements: A benzylic carbocation is a stable carbocation because the positive charge is stabilized by. Draw all reasonable resonance structures for each species. You'll get a detailed solution from a subject matter expert that helps you learn. It is the way of representing molecules in different lewis structures. The molecule can represent a. Draw resonance structures for the following species. Web resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and. 1.49 draw all reasonable resonance structures for each species. Use arrows to show the apparent motion of electron. Include arrows to show electron movement. A benzylic carbocation is a stable carbocation because the positive charge is stabilized by. Web draw all reasonable resonance structures for each species. Determine the relative stability of resonance structures using a set of. The chemical formula of many compounds can have more than. Web be sure to answer all parts. Even more resonance practice problems. Draw the resonance hybrid structures. We always try to draw resonance that has the minimum possible change. For the rat, generate a resonance structure that includes the following. So the resonance structures of the given molecules in the question looks like this. Draw all of the reasonable resonance structures for each of the following molecules. I draw structure draw structure. Web draw all reasonable resonance structures for each species. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web be sure to answer all parts. Determine the location of the double bonds or lone pairs in the molecule. Web resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including. Draw a reasonable resonance structure for the following species. (24 pts) draw all reasonable resonance structures for the following species. Identify the species for which we need to draw resonance structures. Choose the most favorable lewis structure. Even more resonance practice problems. For the rat, generate a resonance structure that includes the following. First, we need to determine the total number of valence electrons for each species. Negative charge on carbon negative. So the resonance structures of the given molecules in the question looks like this. A, g, c, and t. Draw resonance structures for the following species. 1.49 draw all reasonable resonance structures for each species. Determine the relative stability of resonance structures using a set of. Draw the resonance hybrid structures. Draw all reasonable resonance structures for the given species. Use arrows to show the apparent motion of electron.[Solved] Draw all possible resonance structures of each of the
draw all reasonable resonance structures for the following species
Solved draw all reasonable resonance structures for the
[Solved] Draw resonance structures for the following species Course Hero
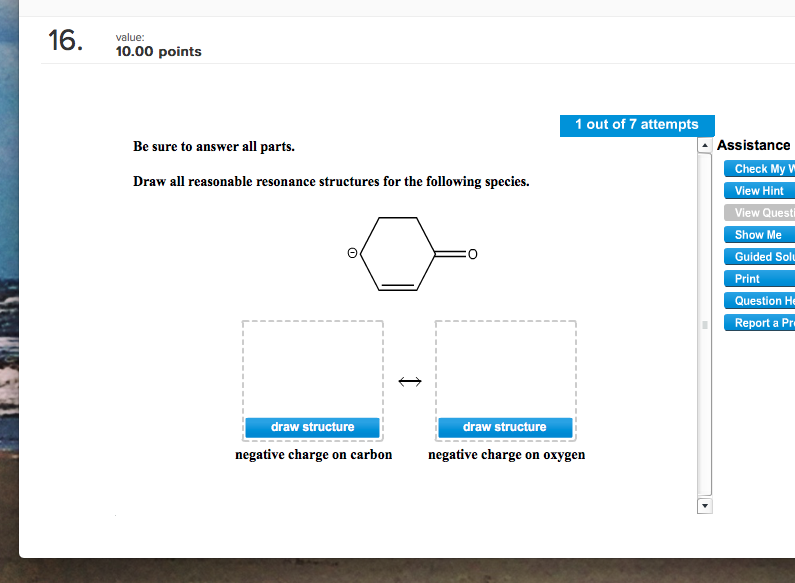
Solved Draw A Reasonable Resonance Structure For The Foll...
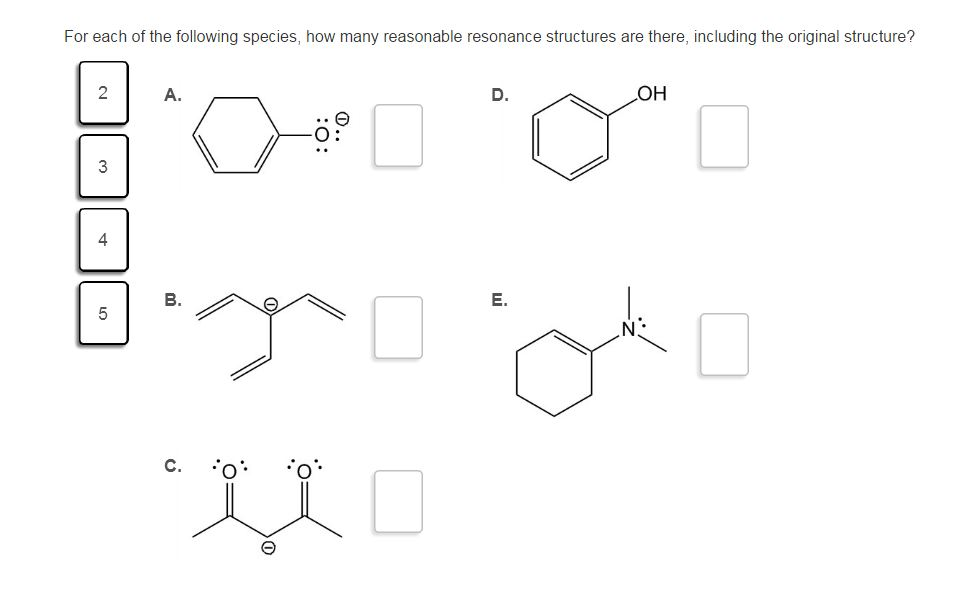
Solved For each of the following species, how many
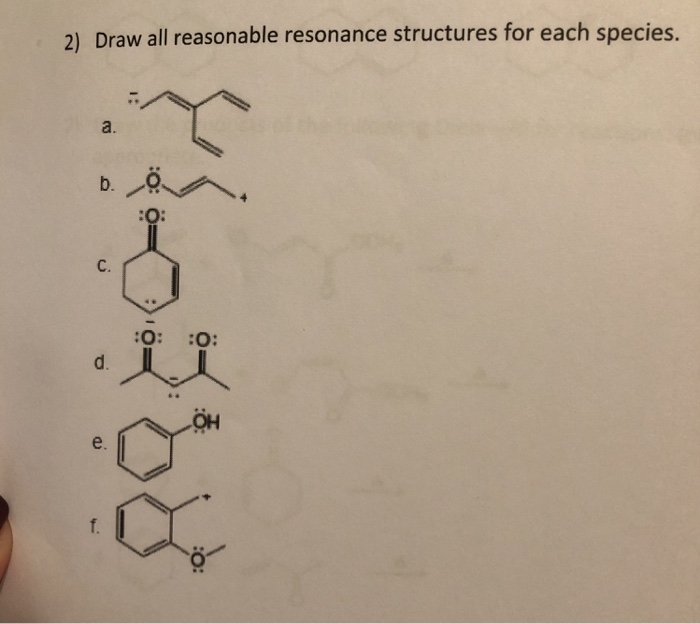
Solved 2) Draw all reasonable resonance structures for each
Draw All Reasonable Resonance Structures For The Following Species at
Solved Draw all of the reasonable resonance structures for
Solved Be sure to answer all parts. Draw all reasonable
Web Be Sure To Answer All Parts.
The Chemical Formula Of Many Compounds Can Have More Than.
Each One Has 6 Additional.
It Is The Way Of Representing Molecules In Different Lewis Structures.
Related Post: