Draw A Picture Of An S Orbital
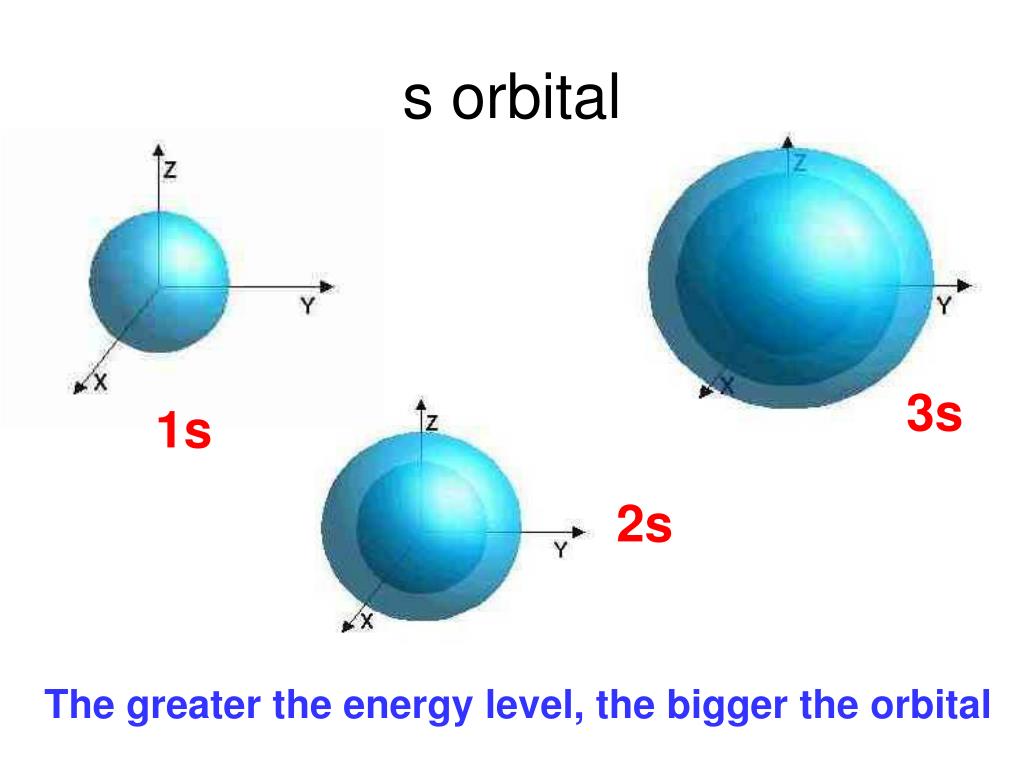
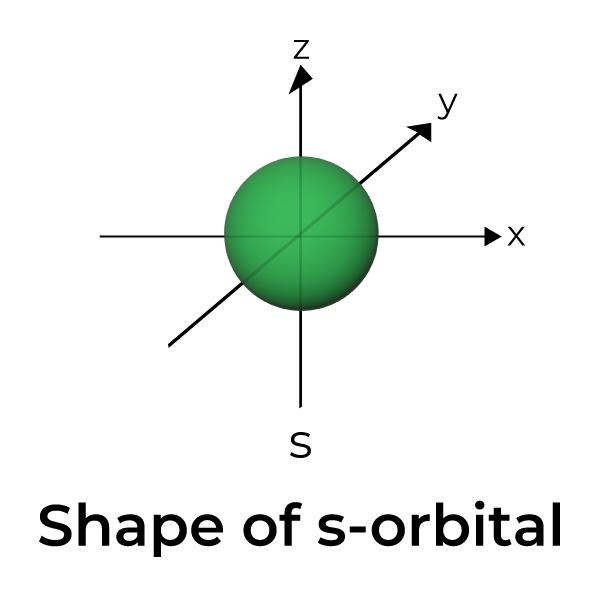
Draw A Picture Of An S Orbital - For an s orbital, draw a circle; Web the molecular orbital theory, initially developed by robert s. They are at a slightly higher level than the 4s. Web for the s orbitals, first draw an arrow pointing up and an arrow pointing down. Web the letter s indicates the shape of the orbital: Imagine shells around the nucleus, that get bigger and bigger. We classified the different orbital into shells and sub shells to distinguish them more easily. In the blue box, draw a picture of an s orbital. Let's learn about the shapes of atomic orbitals in this video lesson.1. Mullikan, incorporates the wave like characteristics of electrons in describing bonding behavior. An orbital is a space where a specific pair of electrons can be found. The orbital shown above is a 2s orbital. For a p orbital, draw a figure eight; The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. Web 1) look at the periodic table to see how many electrons sulfur has. Web the impossibility of drawing orbits for electrons. An orbital is a space where a specific pair of electrons can be found. In molecular orbital theory, the bonding between atoms. For the p orbitals, draw one arrow pointing up on each of the lines first. O what quantum numbers can apply to electrons in sorbitals? Make sure you check here first.what quantum numbers can apply to electrons in s orbitals? Now, draw the second orbital. In the blue box, draw a picture of an s orbital. S orbitalsthere is only 1 s orbital in each s subshell.shape of s. In this case, sulfur has 16 electrons that need to be placed into orbitals. An orbital is a space where a specific pair of electrons can be found. The subscript x,y and z referrers to the axis the orbital is on in 3d. 2) looking at our cheat sheet, draw the orbitals one at a time, adding electrons as you go, until you reach a total of 16 electrons. Imagine shells around the nucleus,. Make sure you check here first.what quantum numbers can apply to electrons in s orbitals? For an f orbital, see below. The real oddity is the position of the 3d orbitals. For a p orbital, draw a figure eight; This orbital will be slightly larger than the first and will be located above and below the first orbital. Web 1) look at the periodic table to see how many electrons sulfur has. Notice that the s orbital always has a slightly lower energy than the p orbitals at the same energy level, so the s orbital always fills with electrons before the corresponding p orbitals. S orbitals are spherically symmetric around the nucleus— they look like hollow balls. They are at a slightly higher level than the 4s. We classified the different orbital into shells and sub shells to distinguish them more easily. Web 1) look at the periodic table to see how many electrons sulfur has. Web so when we say 1s or 3d xz we are orbital in terms of its location in space, and the. Although such drawings show the relative sizes of the orbitals, they do not normally show the spherical nodes in the 2 s and 3 s orbitals because the. Repeat step 3 until all of the orbitals have been drawn. A p orbital consists of two lobes of electron density on either side of the nucleus. The real oddity is the. Web the molecular orbital theory, initially developed by robert s. After completing this section, you should be able to. Imagine shells around the nucleus, that get bigger and bigger. This problem has been solved! For an s orbital, draw a circle; Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. Web the impossibility of drawing orbits for electrons. Below are dot density diagrams, boundary surface diagrams, and a rotating image. Web so when we say 1s or 3d xz we are orbital in. Imagine shells around the nucleus, that get bigger and bigger. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. Web the molecular orbital theory, initially developed by robert s. Web the letter s indicates the shape of the orbital: Web 230313 how to draw shapes of orbitals. Sketch the shapes of s and p orbitals. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For an f orbital, see below. For a p orbital, draw a figure eight; In a typical drawing of orbital, we first plot the radial wave function and the angular part is superimposed. For the p orbitals, draw one arrow pointing up on each of the lines first. This orbital will be slightly larger than the first and will be located above and below the first orbital. In this case, sulfur has 16 electrons that need to be placed into orbitals. In the blue box, draw a picture of an s orbital. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. Make sure you check here first.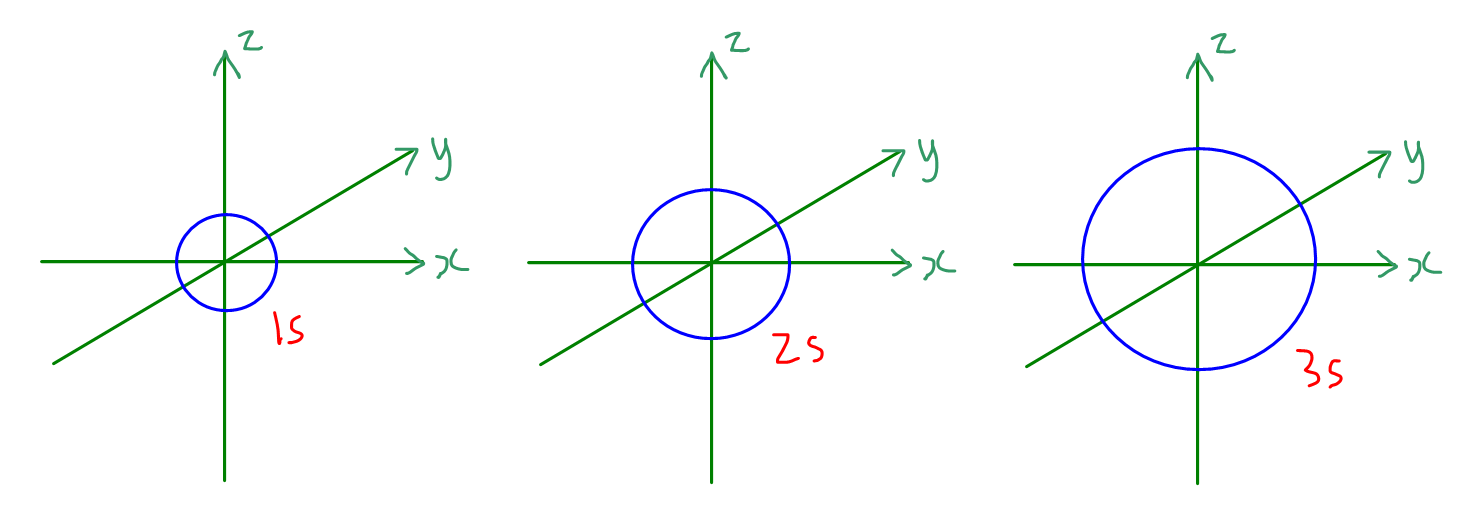
How to Draw Shapes of Orbitals

Biochemistry Glossary Orbitals 2. Shape Draw It to Know It

Atomic orbitals explained polizhuge
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts

Shapes of Atomic Orbitals — Overview & Examples Expii
Shapes of Atomic Orbitals Shape of s, p, d, f Orbitals, FAQs, Examples

8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry

Atoms and Atomic Structure HubPages

12.1.5 Draw the shape of an s orbital and the shapes of the p x , p y
Web So When We Say 1S Or 3D Xz We Are Orbital In Terms Of Its Location In Space, And The Images In Figure \(\Pageindex{1}\) Represents The Shapes Of Some Common Orbitals Where There Is Roughly A 90% Probability Of Finding The Electron That Resides In That Orbital.
Make Certain That You Can Define, And Use In Context, The Key Terms Below.
Repeat Step 3 Until All Of The Orbitals Have Been Drawn.
Make Sure You Check Here First.what Quantum Numbers Can Apply To Electrons In S Orbitals?
Related Post: