Draw A Lewis Structure For Co
Draw A Lewis Structure For Co - This is the co lewis structure: This widget gets the lewis structure of chemical compounds. By the end of this section, you will be able to: Draw lewis structures depicting the bonding in simple. Basis on the periodic table, lewis structure has 4 for carbon and 6 for oxygen. The following procedure can be used to construct lewis electron structures for more complex molecules and. The video covers the basic lewis structures you'll see in an introductor. Write lewis symbols for neutral atoms and ions. Web drawing the co lewis structure involves several steps: Decide which is the central atom in the structure. Count the total number of valence electrons of carbon and oxygen atom. Basis on the periodic table, lewis structure has 4 for carbon and 6 for oxygen. For the co lewis structure, calculate the total number of valence electrons for the co molecule. Carbon is a member of the iva group and has four electrons in its valence shell. Determine. By the end of this section, you will be able to: Web added jun 9, 2014 by webtester in chemistry. The outer (valence) shell configuration of carbon and oxygen atoms. Web lewis structure of carbon monoxide (co) molecule. After determining how many valence. By the end of this section, you will be able to: Web added jun 9, 2014 by webtester in chemistry. For the co structure use the periodic table to find. 592k views 10 years ago co2 lewis, shape, hybridization, polarity,. To determine the total number of valence electrons in co, you. The video covers the basic lewis structures you'll see in an introductor. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. The outer (valence) shell configuration of carbon and oxygen atoms. This widget gets the lewis structure of chemical compounds. Web added jun 9, 2014 by webtester in chemistry. We have 4 valence electrons for carbon and 6 for oxygen, for a total of 10 valence electrons. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. Web lewis structure of carbon monoxide (co) molecule. For the co lewis structure, calculate the total number of valence electrons for the co molecule. Determine the. For the co lewis structure, calculate the total number of valence electrons for the co molecule. Web in the periodic table, oxygen belongs to the via group and has six electrons in its final shell. We have 4 valence electrons for carbon and 6 for oxygen, for a total of 10 valence electrons. Web this video teach you step by. Send feedback | visit wolfram|alpha. Web this video teach you step by step how to draw the lewis structure for co carbon monoxide. Web we are required to draw the lewis structure of the molecule having the chemical formula c o Co formed by adding carbon and oxygen. Web in this video you’ll learn how to draw lewis dot structures. The outer (valence) shell configuration of carbon and oxygen atoms. Drawing the lewis structure for co. Web in the periodic table, oxygen belongs to the via group and has six electrons in its final shell. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. Web in this video you’ll learn how to draw. 592k views 10 years ago co2 lewis, shape, hybridization, polarity,. By the end of this section, you will be able to: Web we are required to draw the lewis structure of the molecule having the chemical formula c o For the co structure use the periodic table to find. Web lewis structure of carbon monoxide (co) molecule. The outer (valence) shell configuration of carbon and oxygen atoms. Count the total number of valence electrons of carbon and oxygen atom. By the end of this section, you will be able to: Oxygen is present in group 16. 125k views 3 years ago. Web we are required to draw the lewis structure of the molecule having the chemical formula c o The video covers the basic lewis structures you'll see in an introductor. Oxygen is present in group 16. Drawing the lewis structure for co. The following procedure can be used to construct lewis electron structures for more complex molecules and. To determine the total number of valence electrons in co, you. For the co lewis structure, calculate the total number of valence electrons for the co molecule. Web drawing the co lewis structure involves several steps: Basis on the periodic table, lewis structure has 4 for carbon and 6 for oxygen. Determine the total number of valence electrons in co. We have 4 valence electrons for carbon and 6 for oxygen, for a total of 10 valence electrons. 592k views 10 years ago co2 lewis, shape, hybridization, polarity,. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells. Draw a skeleton structure in which the other. Web in the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web this video teach you step by step how to draw the lewis structure for co carbon monoxide.
Lewis Structure of CO (Carbon Monoxide) YouTube

How to Draw a Lewis Structure

How do you draw the Lewis structure for CO (Carbon monoxide)?Write

How to Draw the Lewis Dot Structure for Carbon monoxide YouTube

Co Molecule Lewis Structure
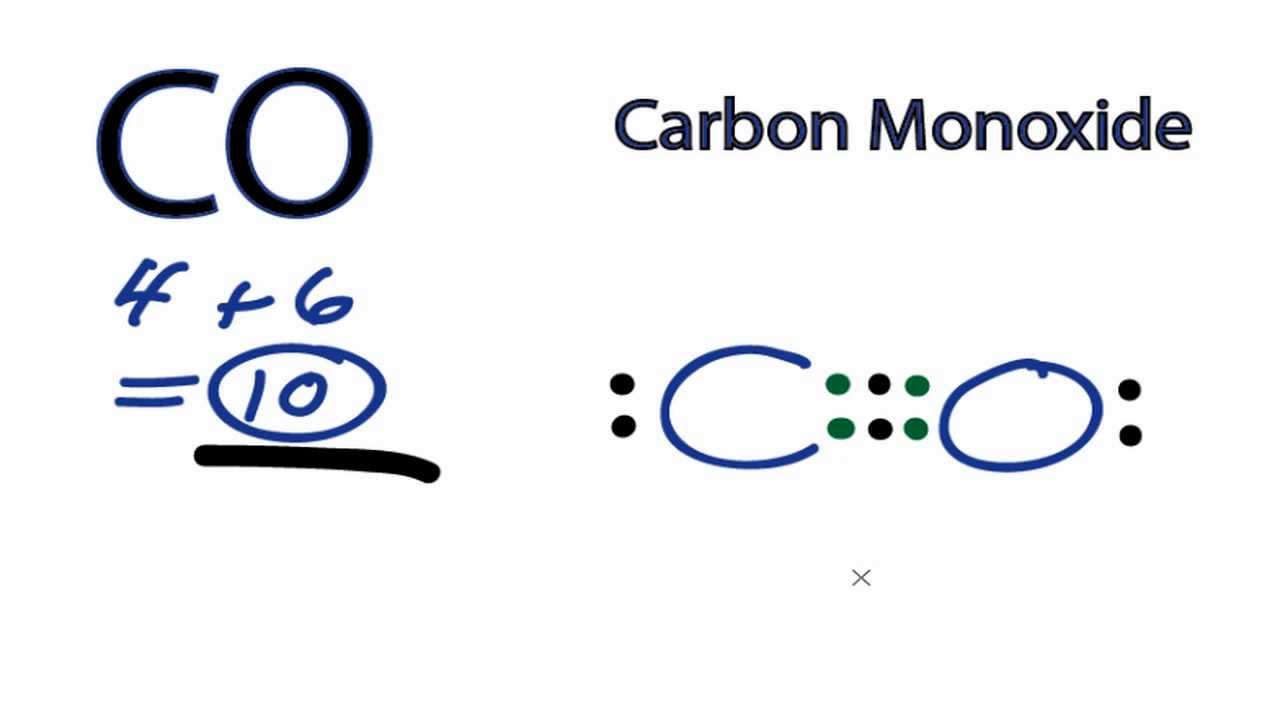
Carbon Monoxide Lewis Structure

Carbon Dioxide Lewis Structure How to Draw the Lewis Structure for

Lewis Dot Structure Definition, Examples, and Drawing
[Solved] 8. Draw the Lewis structures for CO2 and CO, and predict the

CO Lewis Structure, Geometry, and Hybridization Techiescientist
Decide Which Is The Central Atom In The Structure.
Write Lewis Symbols For Neutral Atoms And Ions.
Web Draw Lewis Structures For Covalent Compounds.
Count The Total Number Of Valence Electrons Of Carbon And Oxygen Atom.
Related Post: