Draw A Chiral Alcohol With The Formula C4H10O
Draw A Chiral Alcohol With The Formula C4H10O - The correct option is a 1. The isomers must be alcohols and ethers. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. The three isomers of butanol are possible. The correct combination of names for isomeric alcohols with molecular. Web draw the structures of 4 alcohols with formula c_4h_10o. We know from the formula that these isomers contain no rings or double bonds. Structural formula of these isomers. This problem has been solved! Web the alcohol denaturation process does not chemically change the ethanol molecule. We know from the formula that these isomers contain no rings or double bonds. And butan − 2 − o l. The two alcohols with four carbon atoms in a row are butan − 1 − o l. Web play quiz game > +1 vote. Web how many alcohol with molecular c4h10o are chiral in nature? We know from the formula that these isomers contain no rings or double bonds. Let's start by drawing the. This problem has been solved! Answered jul 12, 2022 by girishgupta (44.4k points) selected jul 13, 2022 by tanishkajain. Correct option is a) possible structures of alcohols with molecular formula c 4h 10o are ch 3butan−1−olc h 2ch 2ch 2ohch 3ch. The three isomers of butanol are possible. The correct option is a 1. Draw one structure per sketcher. This page defines an alcohol, and explains the differences between primary, secondary and tertiary alcohols. Web there are four isomeric alcohols and three isomeric ethers. The other two isomers are. Structural formula of these isomers. See the structural formulas above. The correct combination of names for isomeric alcohols with molecular. And butan − 2 − o l. Web the alcohol denaturation process does not chemically change the ethanol molecule. The correct option is a 1. The two alcohols with four carbon atoms in a row are butan − 1 − o l. Add additional sketchers using the. And butan − 2 − o l. Draw one structure per sketcher. The three isomers of butanol are possible. Web there are four isomeric alcohols and three isomeric ethers. Web the alcohol denaturation process does not chemically change the ethanol molecule. Web draw the structures of 4 alcohols with formula c_4h_10o. Web isomers are compounds that have the same number of atoms as each other, i.e., they have the same empirical formula, but differ in the way the atoms are ordered. The correct combination of names for isomeric alcohols with molecular. This problem has been solved! Correct option is a) possible structures of alcohols with molecular formula c 4h 10o are. There are three possible isomers for alcohols with the formula c4h10o: Web draw a chiral alcohol with the formula c4h10o. Let's start by drawing the. The two alcohols with four carbon atoms in a row are butan − 1 − o l. Add additional sketchers using the. Web draw a chiral alcohol with the formula c4h10o. The three isomers of butanol are possible. Web the alcohol denaturation process does not chemically change the ethanol molecule. Let's start by drawing the. Correct option is a) possible structures of alcohols with molecular formula c 4h 10o are ch 3butan−1−olc h 2ch 2ch 2ohch 3ch 2−. The isomers must be alcohols and ethers. See the structural formulas above. Web 10.1 structure and classification of alcohols. Structural formula of these isomers. Web draw a chiral alcohol with the formula c4h10o. Web isomers are compounds that have the same number of atoms as each other, i.e., they have the same empirical formula, but differ in the way the atoms are ordered. Web there are four isomeric alcohols and three isomeric ethers. Draw one structure per sketcher. Web the alcohol denaturation process does not chemically change the ethanol molecule. Web how many alcohol with molecular c4h10o are chiral in nature? Add additional sketchers using the. Answered jul 12, 2022 by girishgupta (44.4k points) selected jul 13, 2022 by tanishkajain. There are three possible isomers for alcohols with the formula c4h10o: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The other two isomers are. This problem has been solved! The isomers must be alcohols and ethers. The two alcohols with four carbon atoms in a row are butan − 1 − o l. Correct option is a) possible structures of alcohols with molecular formula c 4h 10o are ch 3butan−1−olc h 2ch 2ch 2ohch 3ch 2−. You do not have to consider stereochemistry. Possible structures of alcohols with molecular formula c4h 10o are ch 3 c butan−1−olh 2ch 2ch 2oh ch 3ch 2 − ch3.2. How many alcohols with molecular formula C4H10O are chiral in nature

How many alcohols with molecular formula C4H10O are chiral in nature?
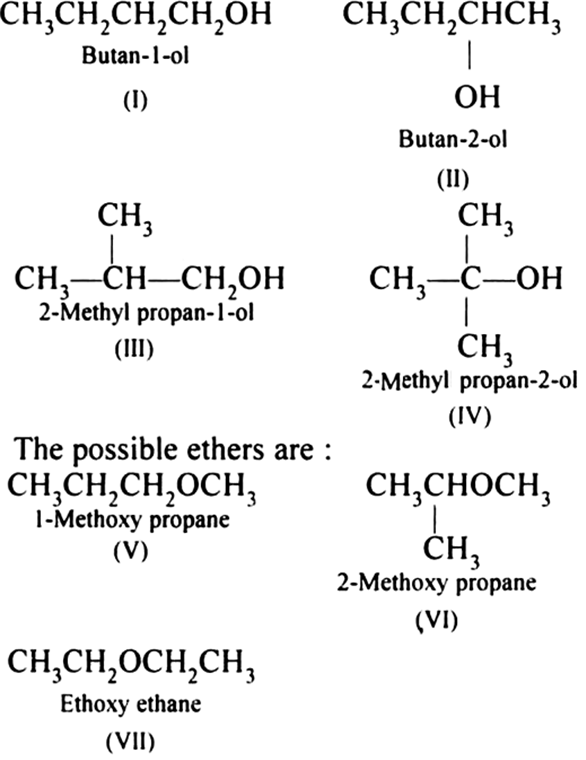
Draw all possible alcohols and ether structures for a compound with
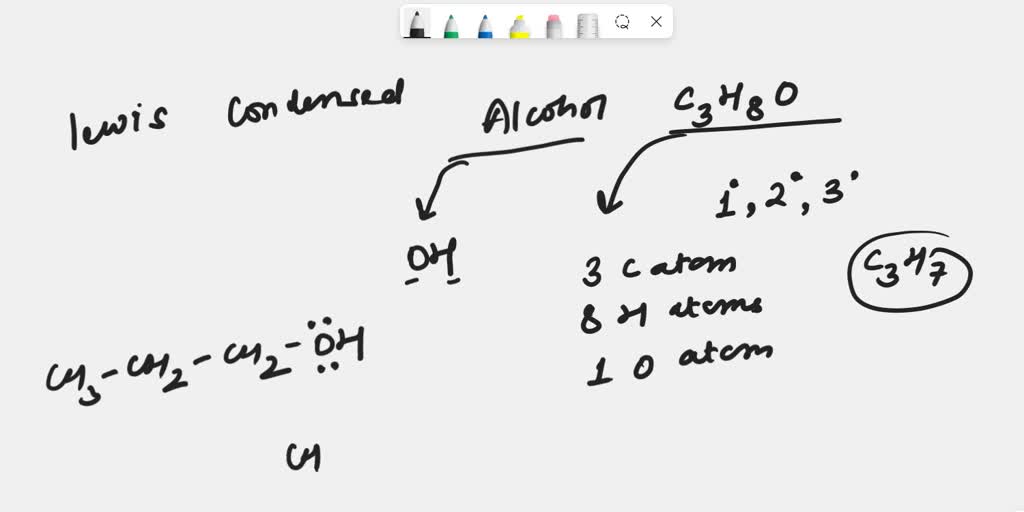
SOLVED Draw Lewis structures and condensed structural formulas for the

Write the structures of the isomers of alcohols with molecular formula

Draw a chiral alcohol with the formula C4H10O.
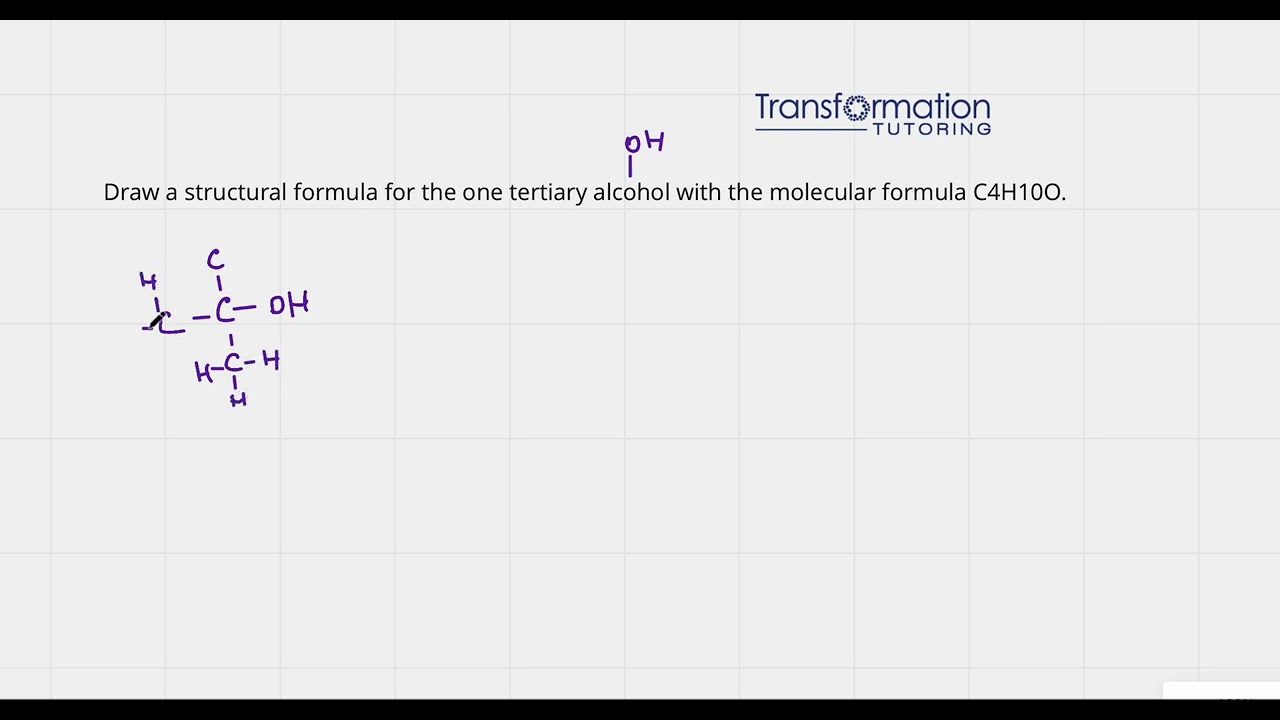
Draw a structural formula for the one tertiary alcohol with the

How many alcohols with molecular formula C4H10O are chiral in nature?
Solved [References] Draw a chiral alcohol with the formula

SOLVED Draw structural formulas for all the alcohols with the formula
Web Play Quiz Game > +1 Vote.
The Correct Option Is A 1.
Structural Formula Of These Isomers.
We Know From The Formula That These Isomers Contain No Rings Or Double Bonds.
Related Post:
![Solved [References] Draw a chiral alcohol with the formula](https://media.cheggcdn.com/media/cfd/cfd71c2a-60ba-40a7-a11c-3b7d73b7cf3e/image)