Butane Drawing
Butane Drawing - You can draw the double bond two ways: The conformational isomers of propane. 1) click on the single bond icon and simply draw another single bond over the existing one between the 1st and 2nd carbons or 2) go the “tool” box and click on the multiple bond icon and Not much more to it than that. Web click the structures and reaction arrows to view the 3d models and animations respectively. Web all about the six key conformations of butane (drawn as newman projections) and comparing their relative energies, using short videos of models. The commonest way to draw structural formulae. The rotation usually results in. However, even a simple molecule like butane can be represented a ton of different ways. For the c4h10 structure use the periodic table to find the total. Web butane is an organic compound with the formula c4h10. The eclipsed form of butane is when the two methyl groups coloured green and blue overlap. The point of this post is to demonstrate a sampling of the many reasonable ways to draw it (along with a few ridiculous ones). This organic chemistry video tutorial provides a basic introduction into. Butane is primarily used as a gasoline mixture, either alone or in a propane mixture. In the case of ethane, conformational changes are very subtle, but in others they are more obvious. The staggered conformation of butane is when both the methyls coloured green and blue are. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at.. College of saint benedict/saint john's university. 23k views 4 years ago. It is also used as a feedstock for ethylene and butadiene production. There are 2 isomers of butane: Web how to draw isomers of butane and butene (gcse) This organic chemistry video tutorial provides a basic introduction into newman. Web draw butane as described before. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. The structures above show the staggered and eclipsed forms of butane. Web depict the anti, gauche, eclipsed and fully eclipsed conformers of butane. The eclipsed form of butane is when the two methyl groups coloured green and blue overlap. Propane is simple to draw as a line diagram. Web look at the models. Web organic chemistry tutorial video showing how to draw the newman projection of butane from the sawhorse projection in both staggered and eclipsed, along with a potential energy diagram. This. Now that we’ve looked at ethane, let’s go to the next higher alkane: There are 2 isomers of butane: Web description stuctural drawings of butane 854px.jpg. Web butane is an organic compound with the formula c4h10. College of saint benedict/saint john's university. 1) click on the single bond icon and simply draw another single bond over the existing one between the 1st and 2nd carbons or 2) go the “tool” box and click on the multiple bond icon and Web a video explanation of how to draw the lewis dot structure for butane, along with information about the compound including formal charges,. In the case of ethane, conformational changes are very subtle, but in others they are more obvious. You can draw the double bond two ways: The commonest way to draw structural formulae. This means we an also identify one unique eclipsed situation. Let's number the carbons along the chain c1, c2, c3 and c4. It is also used as a feedstock for ethylene and butadiene production. This organic chemistry video tutorial provides a basic introduction into newman. For the c4h10 structure use the periodic table to find the total. However, even a simple molecule like butane can be represented a ton of different ways. You can draw the double bond two ways: Web all about the six key conformations of butane (drawn as newman projections) and comparing their relative energies, using short videos of models. Web however, in butane (c4h10, the one in this video), the front and back carbons in the newman projections each have a methyl group, and we use those to identify anti or gauche. For the c4h10 structure. Not much more to it than that. 71k views 10 years ago. Butane is primarily used as a gasoline mixture, either alone or in a propane mixture. Web it’s four carbons in a row. College of saint benedict/saint john's university. Web description stuctural drawings of butane 854px.jpg. Web all about the six key conformations of butane (drawn as newman projections) and comparing their relative energies, using short videos of models. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. 1) click on the single bond icon and simply draw another single bond over the existing one between the 1st and 2nd carbons or 2) go the “tool” box and click on the multiple bond icon and For the c4h10 structure use the periodic table to find the total. 603k views 2 years ago new organic chemistry playlist. Web depict the anti, gauche, eclipsed and fully eclipsed conformers of butane (or a similar compound), using sawhorse representations and newman projections. Web click the structures and reaction arrows to view the 3d models and animations respectively. Just like stick figures of people omit granular details like fingers and toes, line diagrams of molecules omit the placement of hydrogens. The commonest way to draw structural formulae. The eclipsed form of butane is when the two methyl groups coloured green and blue overlap.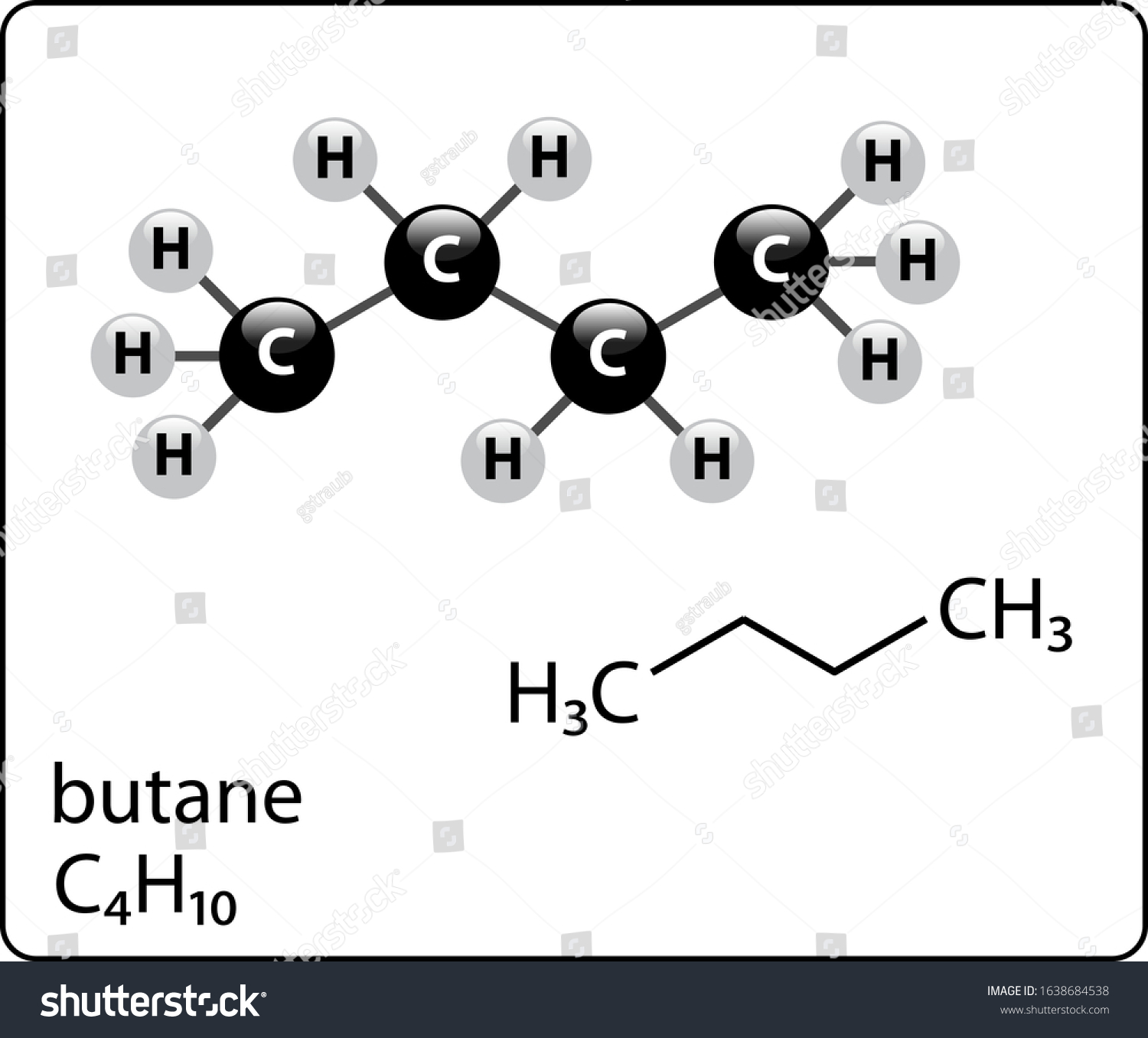
Butane Molecule Structure Ball Stick Stock Vector (Royalty Free

Draw electron dot structure of butane.
Butane molecule is a 3d formula Royalty Free Vector Image
[Solved] Draw butane (C 4 H 10 ). What class of organic molecule is

Butane Molecular Geometry Hybridization Molecular Weight

Butane molecule, illustration Stock Image F030/7874 Science Photo
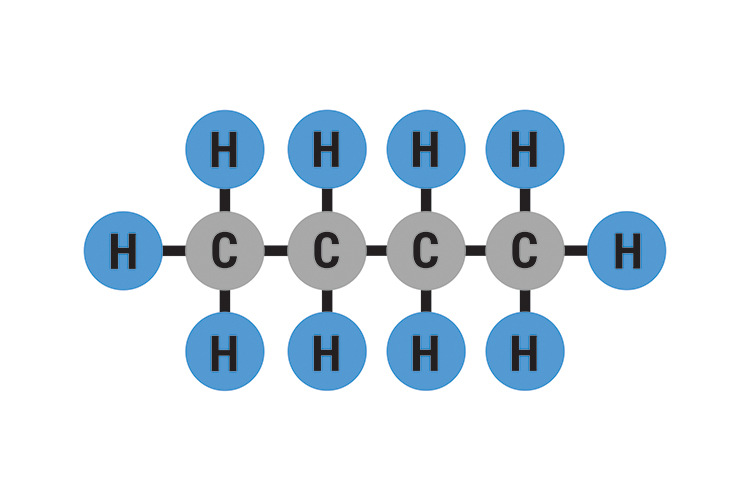
The molecular structure of Butane and formula structure

Butane Molecule Image Royalty Free Vector Image

The illustration of the butane structural formula Stock Vector Image

Butane stock illustration. Illustration of education 83615830
Let's Number The Carbons Along The Chain C1, C2, C3 And C4.
Propane Is Simple To Draw As A Line Diagram.
Butane Is A Highly Flammable, Colorless, Easily Liquefied Gas That Quickly Vaporizes At.
Now That We’ve Looked At Ethane, Let’s Go To The Next Higher Alkane:
Related Post: