Ammonia Drawing
Ammonia Drawing - Nitrogen (n) is in group 15 of the periodic table, which means it has 5 valence electrons. Nh 3 (ammonia) is a commonly tested lewis structure. In adults, high ammonia levels are usually the result of liver damage that causes poor liver function. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. It is clear to understand that the geometrical structure of nh3 will be bent. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. In its concentrated form, it is dangerous and caustic. 50k views 15 years ago. Ammonia is commonly found in nature and is produced through the decay of plant and animal matter. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. (valence electrons are the electrons that are present in the outermost orbit of any. Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. Web submission drawings are used by architects, site engineers or supervisors and others for a number of purposes: Web draw the lewis diagram as below: Web try for free. Ammonia is commonly found in nature and is produced through the decay of plant and animal matter. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: It also is a good example of a molecule with a trigonal prymidal molecular geometry. (valence electrons are the electrons that. Web drawing the lewis structure for nh 3 ( ammmonia) ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. Web drawing the lewis structure for nh 3. Web steps of drawing nh3 lewis structure. The nh 3 chemical name is. Web understanding the nh3 lewis structure is crucial for comprehending. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). Reference range (s) ≤72 umol/l. The billing party has sole responsibility for cpt coding. This is a clip from the complete video:. This inorganic compound has a pungent smell. Web understanding the nh3 lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. 50k views 15 years ago. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). In its concentrated form, it. This inorganic compound has a pungent smell. Nh 3 (ammonia) is a commonly tested lewis structure. Web ammonia testing is typically ordered to diagnose and monitor elevated ammonia levels, also known as hyperammonemia. To develop a design idea into a coherent proposal, to communicate ideas and concepts, to convince clients of the merits of a design, to enable a contractor. Nh 3 (ammonia) is a commonly tested lewis structure. Web ammonia testing is typically ordered to diagnose and monitor elevated ammonia levels, also known as hyperammonemia. It is clear to understand that the geometrical structure of nh3 will be bent. It’s a simple blood test that lets your doctor measure how much ammonia is in your blood. Web understanding the. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair). Web drawing the lewis structure for nh 3 ( ammmonia) ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. This will help you understand the molecule’s electronic structure and bonding. It is. These are arranged in a tetrahedral shape. (valence electrons are the electrons that are present in the outermost orbit of any. Web try for free. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. The arabic numeral above the element's. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: This is a clip from the complete video:. These are. Web steps of drawing nh3 lewis structure. Ammonia tests measure the amount of ammonia in a sample of blood. In its concentrated form, it is dangerous and caustic. Web ammonia testing is typically ordered to diagnose and monitor elevated ammonia levels, also known as hyperammonemia. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: 50k views 15 years ago. These are arranged in a tetrahedral shape. It is a colorless gas with a pungent smell and is lighter than air. This is a clip from the complete video:. Web 3 min read. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. (valence electrons are the electrons that are present in the outermost orbit of any. Reference range (s) ≤72 umol/l. Web try for free. Web ammonia (umol/l) 0 to 14 days: Ammonia is a colorless gas with a chemical formula nh 3.
Ammonia molecule, illustration Stock Image F030/7878 Science
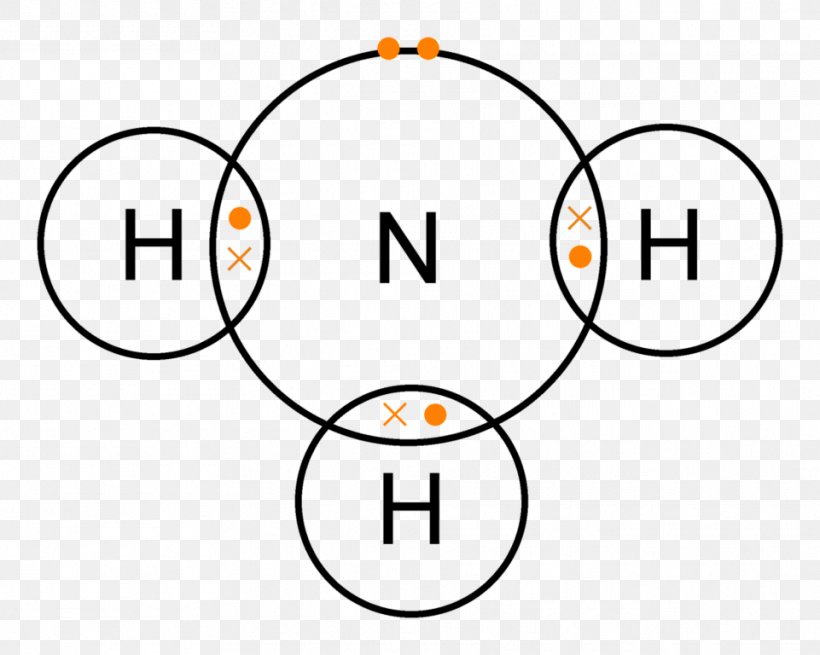
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG

Ammonia molecule model Stock Photo, Royalty Free Image 52584253 Alamy

Ammonia molecule 3d structure Royalty Free Vector Image
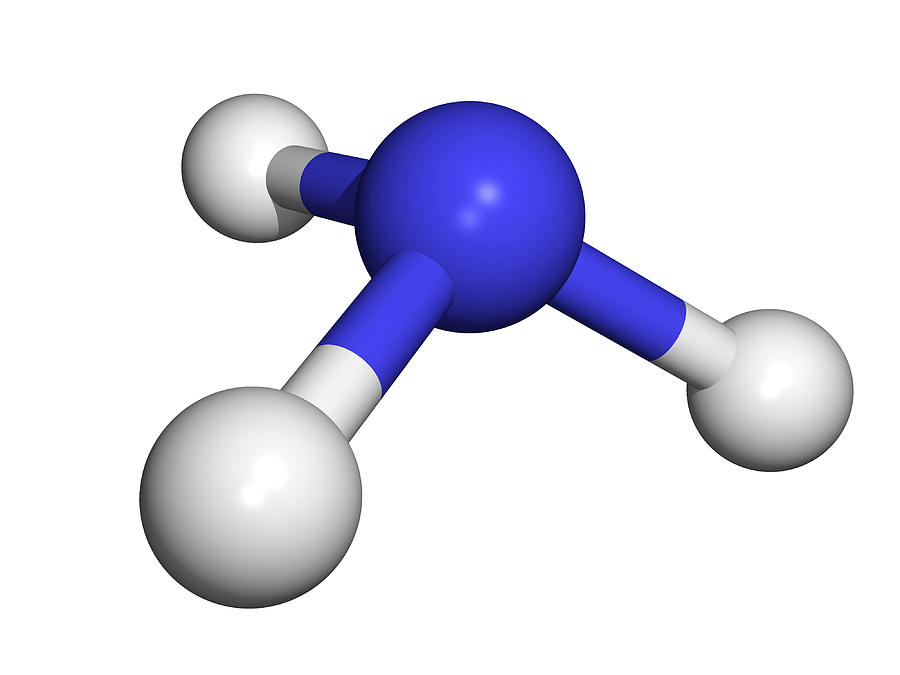
Structure Of Ammonia Molecule
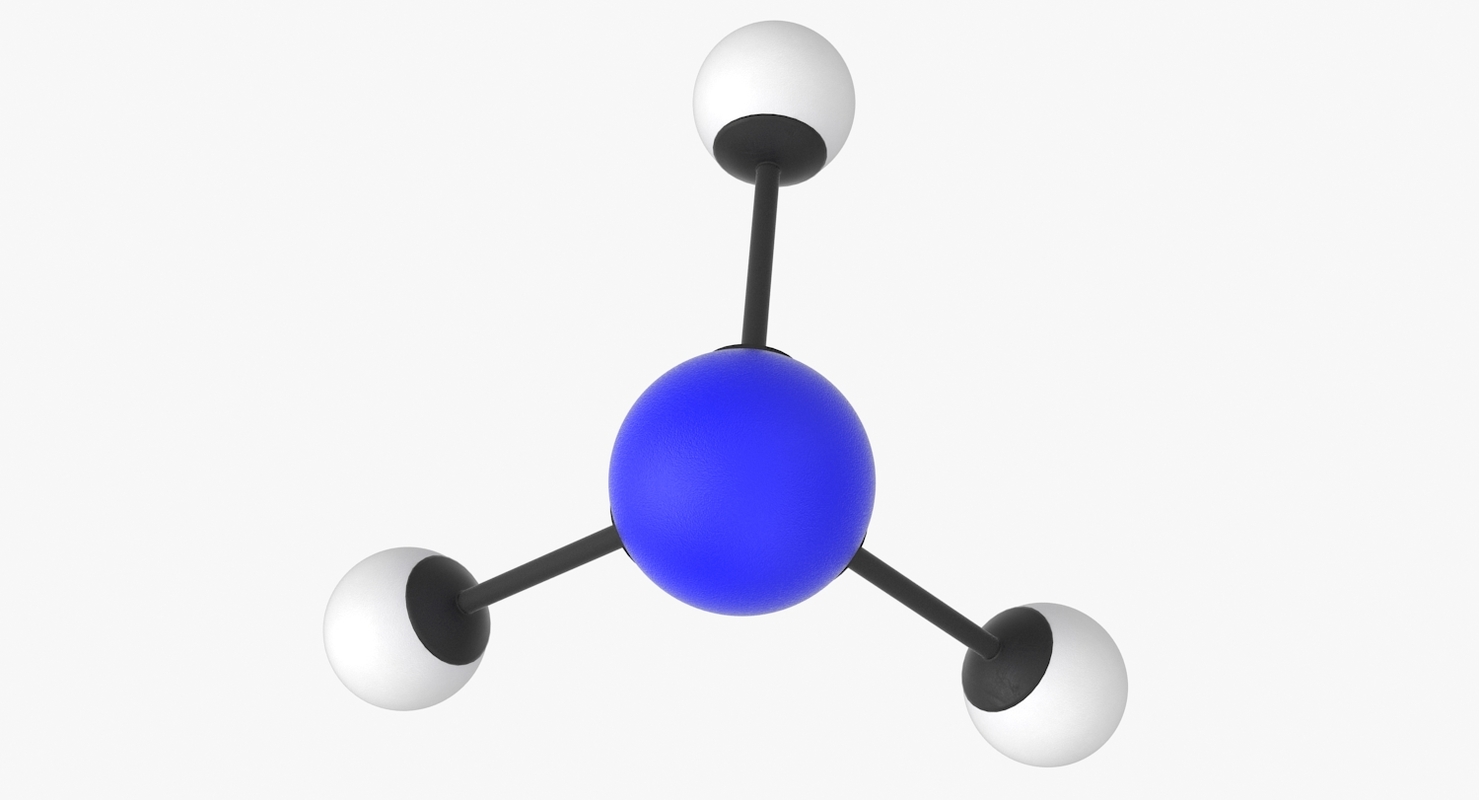
Ammonia molecule 3D model TurboSquid 1418766

Pictogram Van Ammonia Molecule Stock Illustratie Illustration of
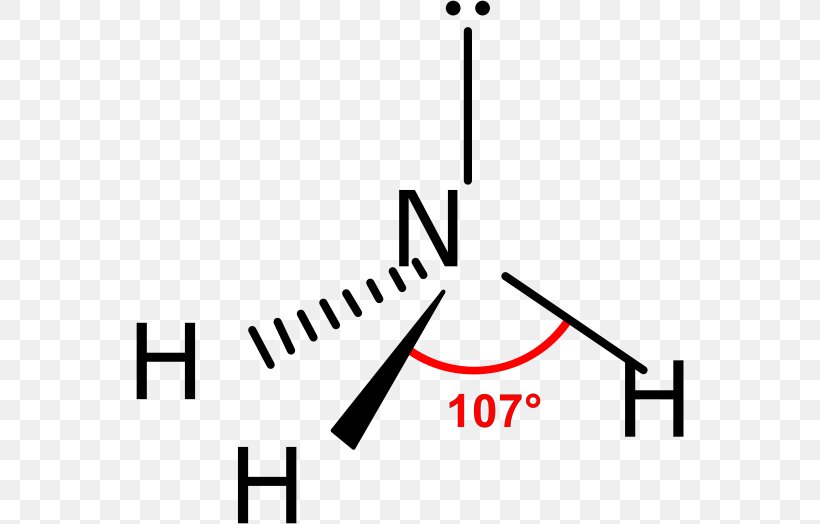
Ammonia Chemical Bond Molecule VSEPR Theory Trigonal Pyramidal
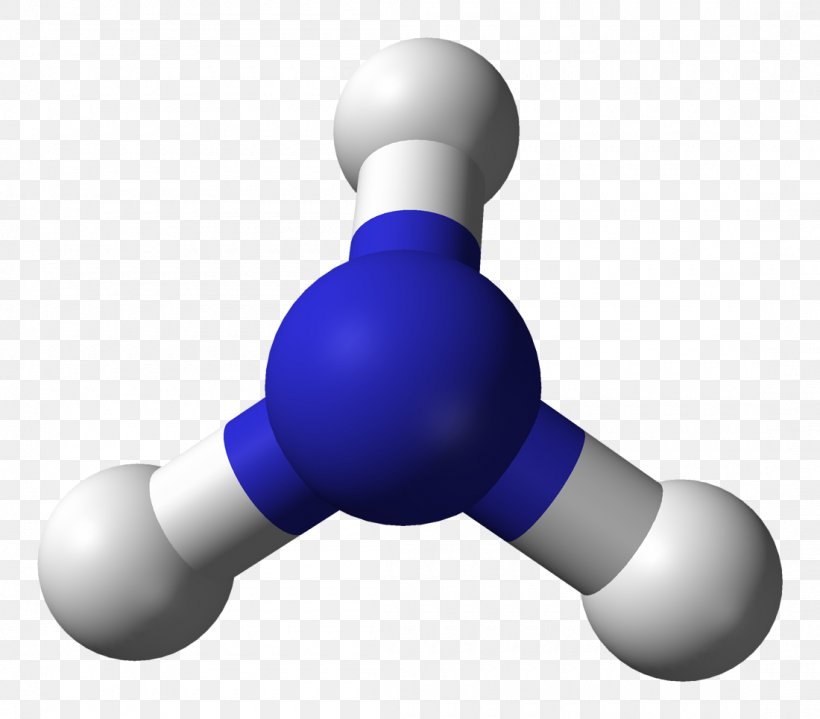
Ammonia Molecule Molecular Geometry Ballandstick Model Lewis
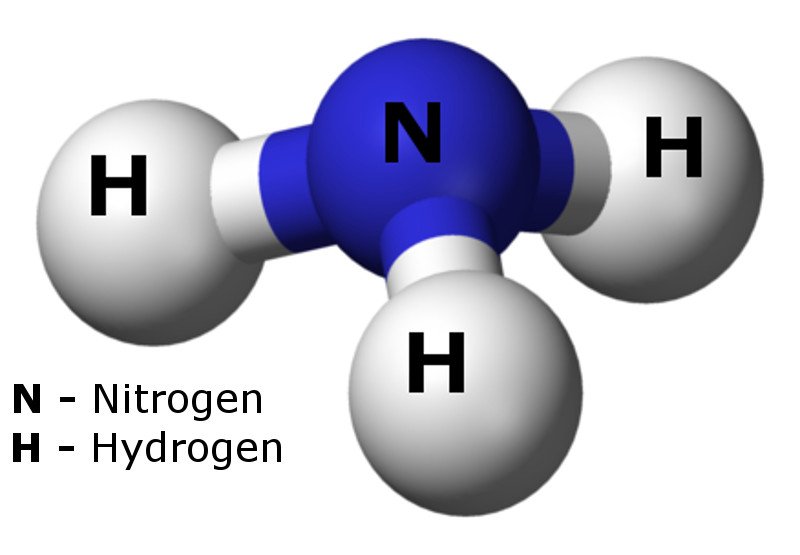
Ammonia Geoviridien
Bacteria In Your Gut And In Your Cells Create Ammonia When Your Body Breaks Down Protein.
Nitrogen (N) Is In Group 15 Of The Periodic Table, Which Means It Has 5 Valence Electrons.
A Simple Method For Drawing The Lewis Structure For Ammonia.
Lewis Structure Of Nh3 Can Be Drawn By Using Valence Electrons Of Nitrogen And Hydrogen Atoms.
Related Post: