4Dxy Orbital Drawing
4Dxy Orbital Drawing - Atomic orbital derived from dft calculations on the corresponding single atoms of the noble gases. And ρ = 2 zeff × r / n (principal quantum number n. Contour surfaces are given at the 90, 80, 70, 60, 50, 40, and 25% probability level of the electron density, describing the spatial volume around a nucleus in which an electron may be found with the corresponding. In tests and exams, you should know to include all these labels without being told for full marks. C) write the shorthand electron configuration of ag+ for the: Here’s the best way to solve it. Question 9) the probability distribution curve for 2s electron appears like that of: Web sketch the 3d orbitals. First year chemistry in the school of chemistry at the university of sydney. 100% (1 rating) share share. Contour surfaces are given at the 90, 80, 70, 60, 50, 40, and 25% probability level of the electron density, describing the spatial volume around a nucleus in which an electron may be found with the. 10) draw the graph of radius of orbit in hydrogen atom as a function of orbit number. Web ρ = 2 zr / n. It looks like a \(2p_z\) orbital combined with an additional doughnut of. First year chemistry in the school of chemistry at the university of sydney. This is the \(3d_{x^2−y^2}\) orbital. In tests and exams, you should know to include all these labels without being told for full marks. 100% (1 rating) share share. Web ρ = 2 zr / n where n is the principal quantum number (4 for the 4 d orbitals) table of equations for the 4d orbitals. Web the five 4 d orbitals. 10) draw the graph of radius of orbit in hydrogen atom as a function of orbit number. Web this problem has been solved! C) write the shorthand. A molecular approach (tro) 7: Use the previous and next icons to see other views. This orbital is related to the 4 dxy orbital (below) by a. This is the \(3d_{x^2−y^2}\) orbital. And ρ = 2 zeff × r / n (principal quantum number n. The total wave function is ψ = y × r with r = radius in bohrs (atomic unit 1 bohr ≅ 0.529 å); 10) draw the graph of radius of orbit in hydrogen atom as a function of orbit number. The shape of atomic orbitals. 2.8k views 8 months ago physical chemistry. 4 d radial distribution function. Contour surfaces are given at the 90, 80, 70, 60, 50, 40, and 25% probability level of the electron density, describing the spatial volume around a nucleus in which an electron may be found with the. A molecular approach (tro) 7: Web a fourth d orbital has lobes lying along the x and y axes; In tests and exams, you. Include the labels of all phases and axes. Hydrogenic atomic orbital derived from the cartesian wave function ψ (see below). Web to sum up, the 3p z orbital has 2 nodes: Web the five 4 d orbitals. A molecular approach (tro) 7: Web since the graph has only one peak, there is no nodal region. How do the 4d orbitals differ from the 3d orbitals? This is the \(3d_{x^2−y^2}\) orbital. The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape: Atomic orbitals 4 d electron density. And ρ = 2 zeff × r / n (principal quantum number n. Web to sum up, the 3p z orbital has 2 nodes: Web since the graph has only one peak, there is no nodal region. Contour surfaces are given at the 90, 80, 70, 60, 50, 40, and 25% probability level of the electron density, describing the spatial. An orbital is the quantum mechanical refinement of bohr’s orbit. Web 230313 how to draw shapes of orbitals. How do the 4d orbitals differ from the 3d orbitals? Web since the graph has only one peak, there is no nodal region. N = 4, l = 2. First year chemistry in the school of chemistry at the university of sydney. Another example is the 5d xy orbital. The 4dxy orbital lies in the xy plane, with lobes along the diagonals of. To understand the 3d representation of electronic orbitals. Web ρ = 2 zr / n where n is the principal quantum number (4 for the 4 d orbitals) table of equations for the 4d orbitals. Atomic orbital derived from dft calculations on the corresponding single atoms of the noble gases. Here’s the best way to solve it. Web this problem has been solved! The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape: The shape of atomic orbitals. 100% (1 rating) share share. C) write the shorthand electron configuration of ag+ for the: 2.8k views 8 months ago physical chemistry. Question 9) the probability distribution curve for 2s electron appears like that of: Include the labels of all phases and axes. Zeff is the effective nuclear charge;[Solved] draw the cross section of a 4dxy orbital Course Hero
[Solved] draw the 4dxy orbital and identify all nodes (radial and
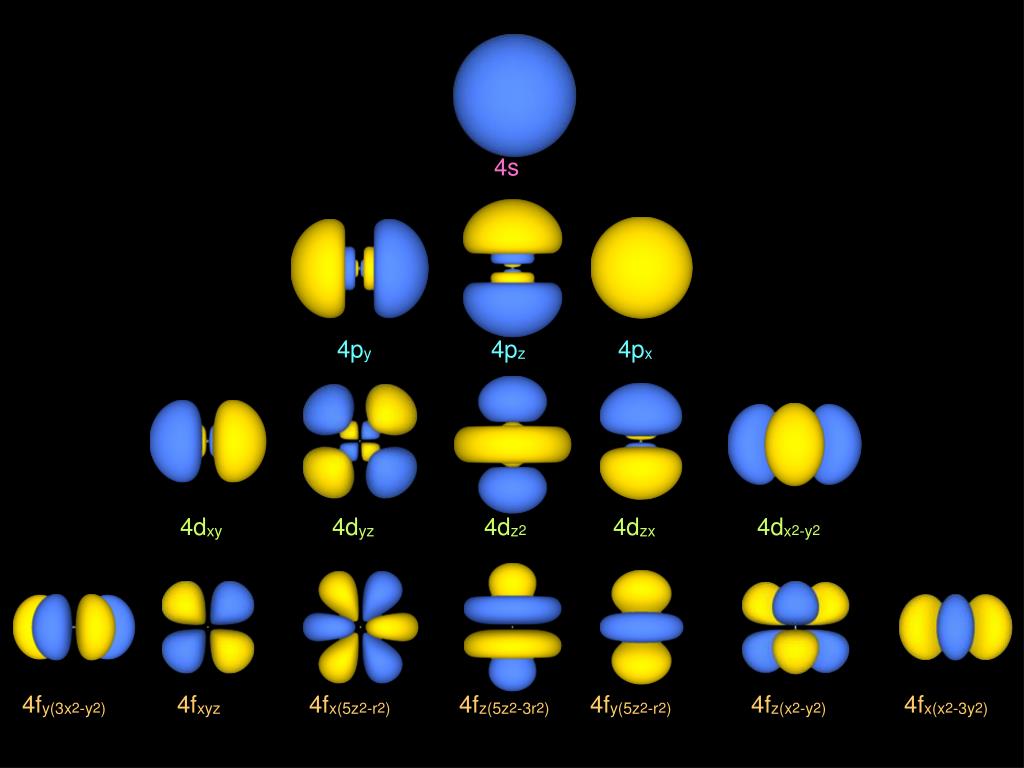
PPT Atomic orbital & Hydrogenatom wave function PowerPoint
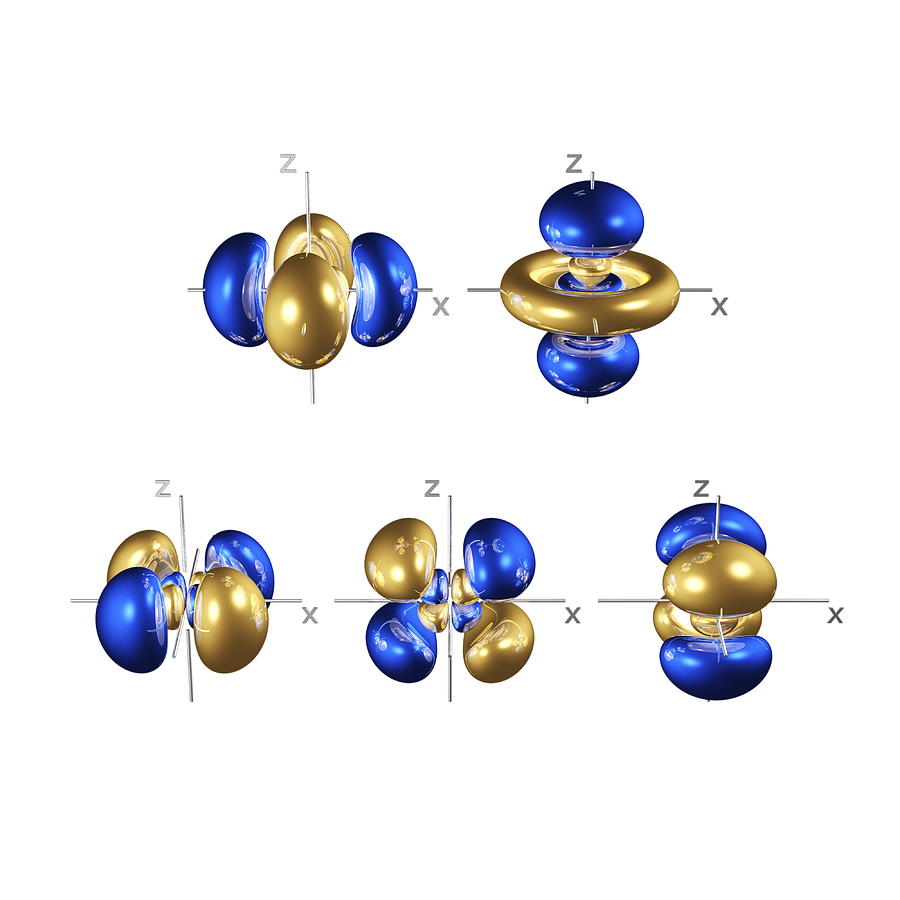
4d Electron Orbitals Photograph by Dr Mark J. Winter Pixels
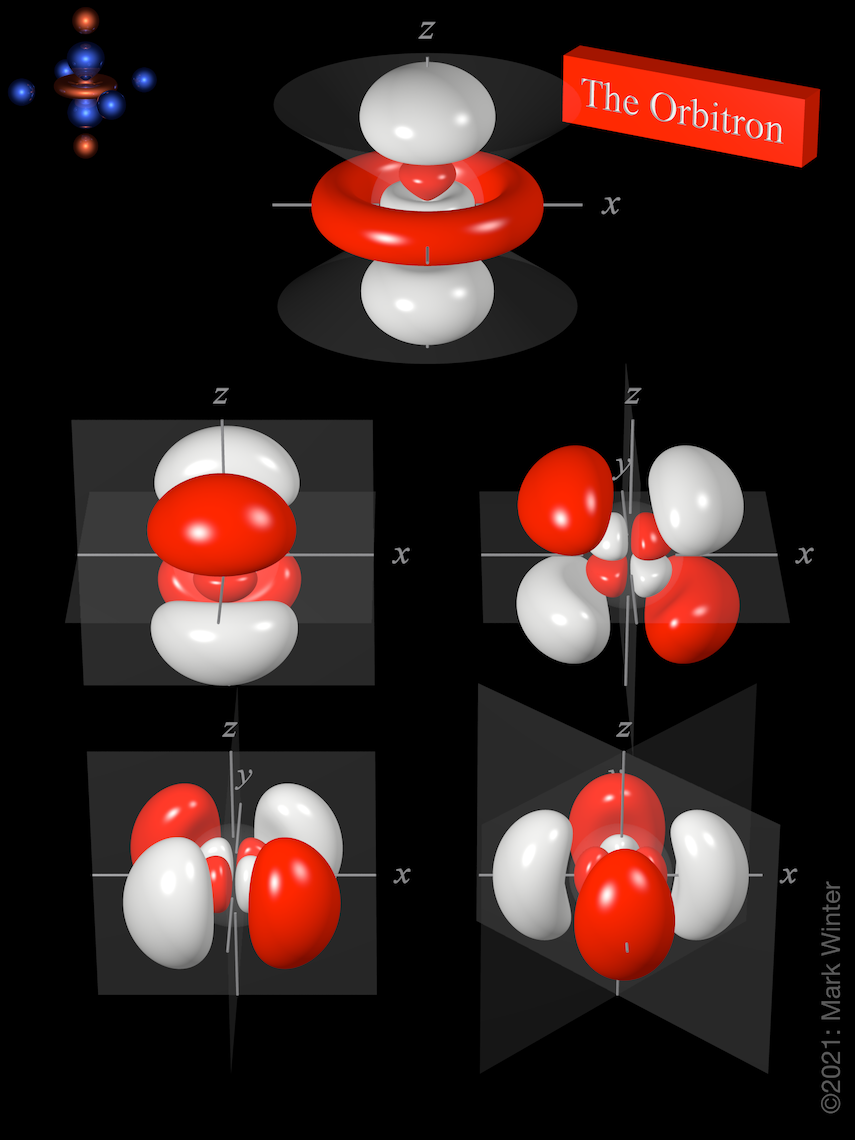
The Orbitron 4d atomic orbitals

Make a sketch of the five 4d orbitals. Describe how the orbitals

The shape of the 4d orbital Chemistry X YouTube
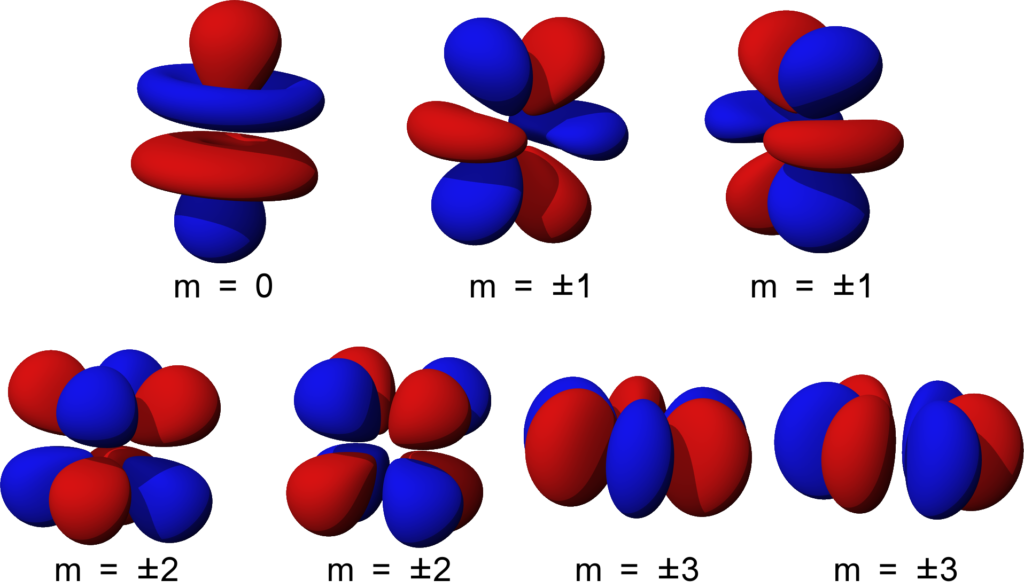
Shapes of Orbitals and their Types Chemistry Skills
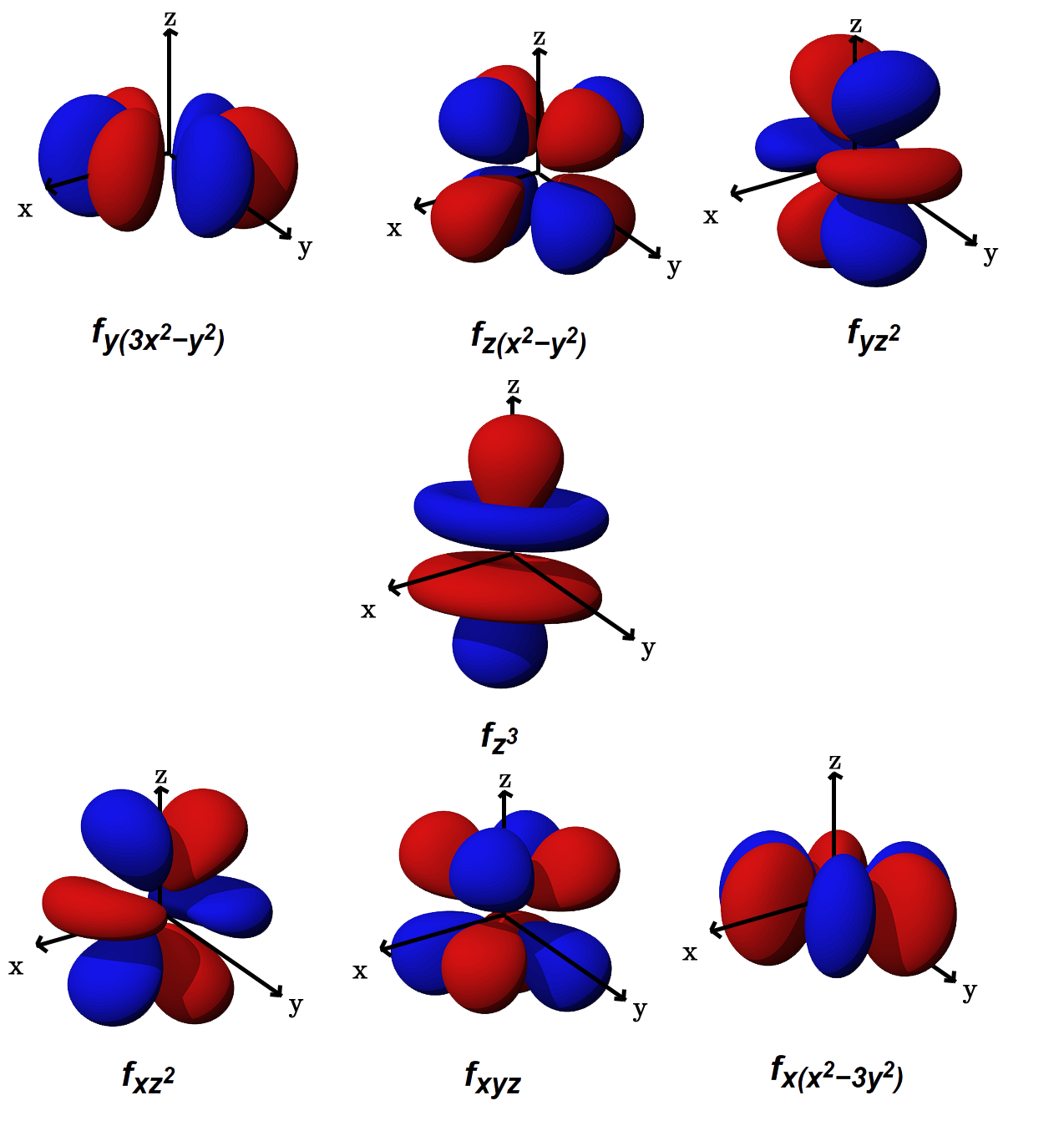
Classify these atomic orbitals innovativehrom
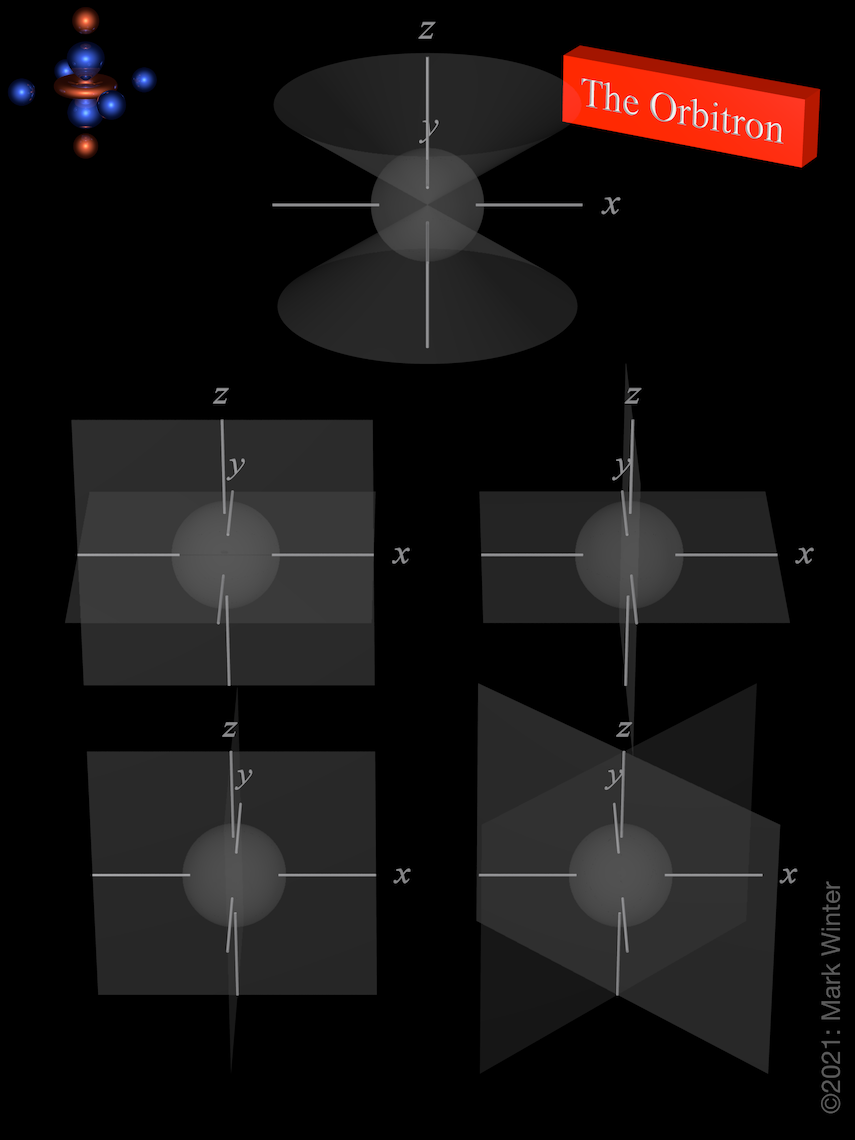
The Orbitron 4d atomic orbitals
And Ρ = 2 Zeff × R / N (Principal Quantum Number N.
Label Any Appropriate Axes As Well As Angular And Radial Nodes.
Web Since The Graph Has Only One Peak, There Is No Nodal Region.
In All Cases The Red Zones Are Where The 4 D Wave Function Has Positive Values.
Related Post: